Abstract
Background
Immune checkpoint inhibitors that target the programmed cell death protein 1 (PD-1) receptor induce a response in only a subgroup of patients with metastatic melanoma. Previous research suggests that transforming growth factor beta signaling and a collagen-rich peritumoral stroma (tumor fibrosis), may negatively interfere with the interaction between T cells and tumor cells and thereby contribute to resistance mechanisms by immune-exclusion, while increased tumor infiltration of M1-like macrophages enhances T cell activity. Hence, the current study aimed to assess the relationship between blood-based markers of collagen or vimentin turnover (reflecting M1 macrophage activity) and clinical outcome in patients with metastatic melanoma after PD-1 inhibition.
Methods
Patients with metastatic melanoma who were treated with anti-PD-1 monotherapy between May 2016 and March 2019 were included in a prospective observational study. N-terminal pro-peptide of type III collagen (PRO-C3) cross-linked N-terminal pro-peptides of type III collagen (PC3X), matrix metalloprotease (MMP)-degraded type III (C3M) and type IV collagen (C4M), granzyme B-degraded type IV collagen and citrullinated and MMP-degraded vimentin (VICM) were measured with immunoassays in serum before (n=107), and 6 weeks after the first administration of immunotherapy (n=94). The association between biomarker levels and overall survival (OS) or progression-free survival (PFS) was assessed.
Results
Multivariate Cox regression analysis identified high baseline PRO-C3 (Q4) and PC3X (Q4) as independent variables of worse PFS (PRO-C3: HR=1.81, 95% CI=1.06 to 3.10, p=0.030 and PC3X: HR=1.86, 95% CI=1.09 to 3.18, p=0.023). High baseline PRO-C3 was also independently related to worse OS (HR=2.08, 95% CI=1.06 to 4.09, p=0.035), whereas a high C3M/PRO-C3 ratio was related to improved OS (HR=0.42, 95% CI=0.20 to 0.90, p=0.025). An increase in VICM (p<0.0001; in 56% of the patients) was observed after 6 weeks of treatment, and an increase in VICM was independently associated with improved OS (HR=0.28, 95% CI=0.10 to 0.77, p=0.014).
Conclusions
Blood-based biomarkers reflecting excessive type III collagen turnover were associated with worse OS and PFS after PD-1 inhibition in metastatic melanoma. Moreover, an increase in VICM levels after 6 weeks of treatment was associated with improved OS. These findings suggest that type III collagen and vimentin turnover contribute to resistance/response mechanisms of PD-1 inhibitors and hold promise of assessing extracellular matrix-derived and stroma-derived components to predict immunotherapy response.
Keywords: immunotherapy, costimulatory and inhibitory T-cell receptors, melanoma, immune evation, tumor microenvironment
Background
Over the last decade, immune checkpoint inhibitors (ICIs) have improved the overall survival (OS) of patients with metastatic cancer. Monoclonal antibodies directed to the programmed cell death protein 1 (PD-1) receptor have emerged as frontline therapies for cancers such as metastatic melanoma. However, there is an unmet need for biomarkers to guide treatment decisions, as the efficacy of ICIs is highly variable across individual patients. Although the expression of its ligand programmed death-ligand 1 (PD-L1) has been positively associated with clinical outcome in metastatic melanoma, the predictive performance remains limited, which may partly be explained by tumor heterogeneity.1 Similarly, other biomarkers, such as CD8+ T cell infiltration and tumor mutational burden, individually or combined, have been considered as biomarkers.2 3 Interestingly, recent evidence suggests that transforming growth factor beta (TGF-β), cancer-associated fibroblasts (CAFs) and immune-exclusion in tumor tissue are associated with worse clinical outcome after PD-L1 or PD-1 inhibition in solid cancer.4–6 Those immune-excluded tumors are characterized by a collagen-rich peritumoral stroma (tumor fibrosis) that blocks the interaction between CD8+ T cells and tumor cells.4–6 The main collagens of the interstitial matrix (type I and III collagen) and the basement membrane (type IV collagen) are upregulated in immune-excluded tumors that are accompanied by a peritumoral location of CD8+ T cells.7 Moreover, components of proteolytic extracellular matrix (ECM) turnover appear to affect the response mechanisms to ICIs by regulating steps in the cancer-immunity cycle.8 Melanoma is characterized by a highly reactive and fibrotic tumor stroma.9 10 Increased stroma activity leads to increased matrix metalloproteinase (MMP)-mediated collagen degradation resulting in the generation and release of small protein fragments containing specific protease-generated neo-epitopes into the blood circulation.11 12 These ECM protein fragments can be used as non-invasive biomarkers measured in blood, and may be used to assess processes such as MMP-mediated collagen degradation and collagen formation and cross-linking by evaluating its blood-based signature.12 This has been demonstrated by our previous study showing that biomarkers measuring N-terminal pro-peptide of type III collagen (PRO-C3) or MMP-degraded type IV collagen (C4M) are associated with worse clinical outcome after cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) ICIs in patients with metastatic melanoma.13 The two collagen-derived biomarkers assess different components of the ECM in the tumor microenvironment (TME) by reflecting a dense stromal interstitial matrix and fibrotic activity (PRO-C3) and degradation of the basement membrane (C4M).14
The current study aimed to investigate the relationship of tissue-derived biomarkers and clinical outcome after PD-1 inhibition in patients with metastatic melanoma. Protein fragments reflecting type III collagen formation (PRO-C3) and cross-linking (PC3X), MMP-degraded type III (C3M) and IV collagen (C4M), granzyme B-degraded type IV collagen (C4G) and citrullinated and MMP-degraded vimentin (VICM) were measured in serum obtained before and 6 weeks after the first administration of PD-1 ICIs. Moreover, the dynamics of collagen turnover and VICM during treatment were assessed.
Methods
Study design and data collection
Patients with metastatic melanoma who were treated with anti-PD-1 monotherapy (pembrolizumab or nivolumab) at the Erasmus MC Cancer Institute (Rotterdam, The Netherlands) were prospectively included in the MULTOMAB immune-monitoring trial (Dutch Trial Registry number NL6828) between May 2016 and March 2019, with a mean follow-up time of 1.8 years (SD: 10 months). Pembrolizumab was administered as a 3 weekly infusion of 2 mg/kg, and nivolumab as a 2 weekly infusion of 3 mg/kg. Patients who were treated with a prior line of immunotherapy or who received combination ICI therapy were excluded. Serum samples drawn at baseline and after 6 weeks (median 6, IQR 6–9 weeks) of treatment were available for the assessment of selected ECM biomarkers. All biomarker assays were performed blinded to clinical outcome and subject numbers were assigned to patients. The biomarker analyses were performed in August 2019. Progression-free survival (PFS) was defined as the time from the first administration of PD-1 ICI until progressive disease (PD; based on Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST V.1.1))15 or death, whichever occurred first. OS was defined as the time from the first administration of PD-1 ICI until death. After treatment initiation, radiological evaluation by CT was usually performed every 3 months. For the determination of the best overall response (BOR) by RECIST V.1.1, a minimum duration of 90 days for stable disease (SD) was required. Confirmation of partial response (PR) or complete response (CR) was not required.
Assessment of biomarkers of extracellular matrix turnover in serum
Levels of ECM biomarkers were assessed in duplicates in serum samples using immunoassays (Nordic Bioscience, Herlev, Denmark) as previously described.7 In short, type III collagen formation were measured with a competitive ELISA targeting the PRO-C3. Moreover, type III collagen formation and cross-linking were measured with a sandwich ELISA targeting PC3X. C4G was measured with a competitive electrochemiluminescence assay. Lastly, C3M, C4M and VICM were measured with competitive ELISAs.
Statistical analysis
First, patient survival (PFS and OS) was visualized by the Kaplan-Meier approach and log-rank tests were used to determine differences between the curves after stratification of patients into quartile groups based on biomarker levels. Kaplan-Meier survival curves were also used to analyze survival outcomes in patients with a low type III collagen degradation to formation (C3M/PRO-C3) ratio (<1) compared with a high C3M/PRO-C3 ratio (>1). Moreover, survival outcomes were analyzed in patients with an evident increase in biomarkers (>20%) at week 6 compared with baseline versus the rest of the patients who did not have an evident increase. The association with survival (PFS and OS) was further analyzed using univariate and multivariate Cox regression analysis. Correction for potential confounding factors was performed, variables with a p value of ≤0.05 were considered for multivariate analyses. Test variables (ECM biomarkers) were included as continuous variable and stratified by the 75th percentile cut-off. Second, to study the association of baseline ECM biomarker levels with BOR, patients with PD were compared with patients with SD/PR/CR and analyzed using the Mann-Whitney U test. Lastly, the dynamics of ECM biomarkers were studied by determining the difference over time of individual ECM biomarkers, indicating an increase or decrease comparing baseline levels with levels during therapy using the Wilcoxon matched-pairs rank test. A Pearson’s correlation (two-tailed) was used to examine biomarker correlations, as well as the correlations of baseline biomarker levels with lactate dehydrogenase (LDH). Analyses were performed using MedCalc (V.16.8.4), IBM SPSS Statistics V.24.0.0.1 (Chicago, Illinois, USA) and Graphpad Prism V.8.3.0 (Graphpad Software). A p value of p<0.05 was considered to be statistically significant.
Results
Serum from a total of 107 patients with metastatic melanoma at baseline and paired serum from 94 patients (88% of all patients) that was obtained 6 weeks (median 6, IQR 6–9 weeks) after PD-1 ICI therapy was available for analysis. The baseline patient characteristics including demographic and clinical data are shown in table 1. Patients generally received PD-1 ICIs as first-line treatment shortly after diagnosis (82% of all patients), and therefore generally have a good performance status (WHO PS 0 or 1: 92% of all patients). All 107 patients were evaluable for the OS analysis, with a median OS of 36.2 months. Three patients (3%) were not evaluable for determination of BOR, that is, because of early clinical progression, resulting in a disease control rate (the proportion of patients with a BOR of SD, PR or CR) of 63.5%. The serum levels of the six different ECM biomarkers were evaluated at baseline: PRO-C3, PC3X, C4G, C3M, C4M and VICM. The distribution of those baseline measurements is shown in online supplemental figure 1. For all six ECM biomarkers, there was a right-skewed distribution of data.
Table 1.
Patient characteristics
| Patient factor | N (%) |
| Gender | |
| Male | 62 (58%) |
| Female | 45 (42%) |
| WHO performance | |
| 0 | 69 (64%) |
| 1 | 30 (28%) |
| 2 | 2 (2%) |
| Unknown | 6 (6%) |
| Treatment regimen | |
| Nivolumab | 62 (58%) |
| Pembrolizumab | 45 (42%) |
| Prior systemic treatment lines | |
| 0 | 88 (82%) |
| 1 | 18 (17%) |
| 2 | 1 (1%) |
| Brain metastases | |
| Present at baseline | 18 (17%) |
| Absent at baseline | 48 (45%) |
| No screening performed | 41 (38%) |
| BOR | |
| CR | 15 (14%) |
| PR | 38 (35.5%) |
| SD | 13 (12%) |
| PD | 38 (35.5%) |
| Not evaluable | 3 (3%) |
| Continuous variables | Median (IQR) |
| Age | 66 (55–73) |
| LDH at baseline | 216 (183–306) |
BOR, best overall response; CR, complete response; LDH, lactate dehydrogenase; PD, progressive disease; PD, partial response; SD, stable disease.
jitc-2020-001193supp002.pdf (305.5KB, pdf)
Baseline levels of PRO-C3 and PC3X are negatively associated with survival outcomes when assessed by Kaplan-Meier method
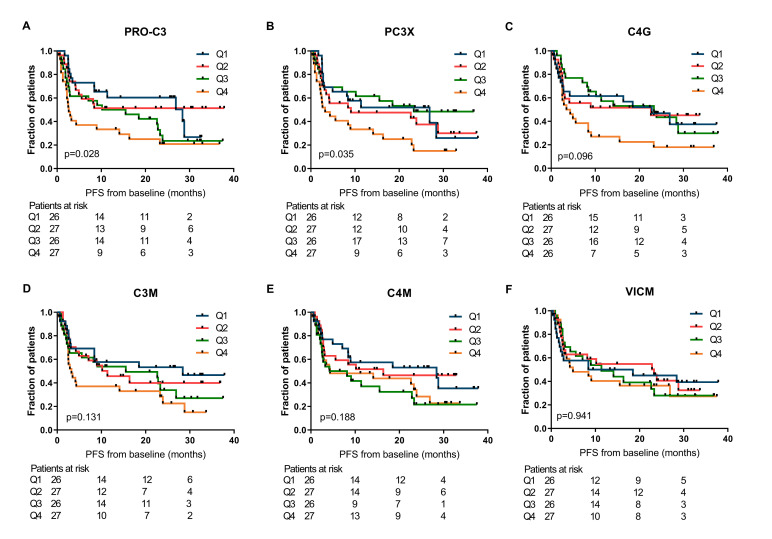
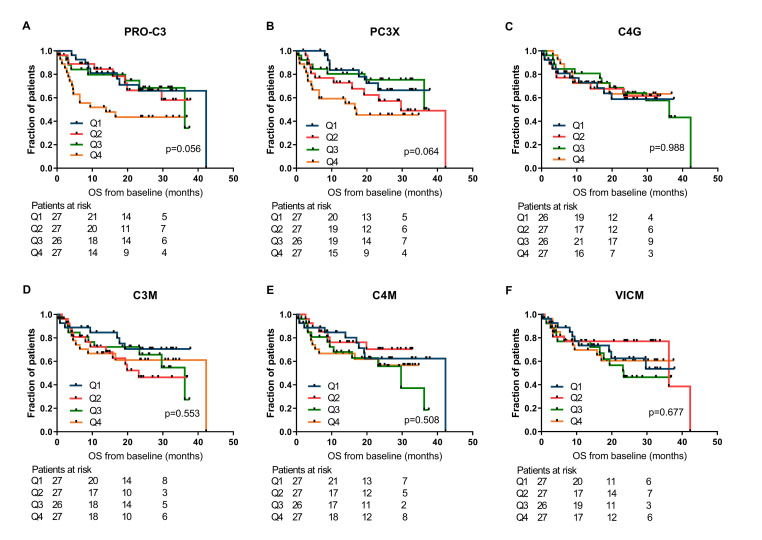
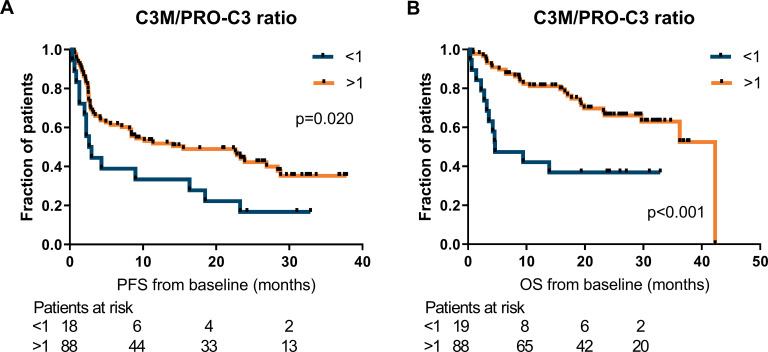
First, the relationship between ECM-derived biomarkers at baseline and clinical outcome after PD-1 inhibition in patients with metastatic melanoma were investigated by the Kaplan-Meier method. For PRO-C3 and PC3X, statistically significant differences among PFS curves were observed across the quartiles of biomarker levels (median PFS of PRO-C3 Q1: 28.4 months, Q2: not reached, Q3: 12.9 months and Q4: 2.6 months, p=0.028 and median PFS of PC3X Q1: 26.9 months, Q2: 9.1 months, Q3: 23.3 months and Q4: 3.2 months, p=0.035; figure 1A, B). Similarly, the association of ECM biomarkers with OS was assessed (figure 2), resulting in a corresponding trend for PRO-C3 and PC3X (median OS of PRO-C3 Q1: 42.3 months, Q2: not reached, Q3: 36.2 months and Q4: 13.9 months, p=0.056 and median OS PC3X Q1: not reached, Q2: 29.7 months, Q3: not reached and Q4: 17.0 months, p=0.064; figure 2A, B). The baseline levels of C4G, C3M, C4M or VICM were not significantly associated with PFS or OS (figures 1 and 2). Of note, PRO-C3 and PC3X levels were moderately correlated with each other at baseline (Pearson’s r=0.60, p<0.0001), while C3M and C4M were strongly correlated at baseline (Pearson’s r=0.86, p<0.0001; online supplemental table 1). Interestingly, patients with a high C3M/PRO-C3 ratio (>1) at baseline had improved PFS (median PFS: 15.5 vs 2.8 months, p=0.020) and OS (median OS: 42.3 vs 4.6 months, p<0.001) compared with patients with a low C3M/PRO-C3 ratio (<1) (figure 3A, B).
Figure 1.
Association of extracellular matrix (ECM) biomarkers measured at baseline and progression-free survival (PFS). Kaplan-Meier plots demonstrating the association of ECM biomarkers with PFS for patients with metastatic melanoma. (A) PRO-C3, (B) PC3X, (C) C4G, (D) C3M, (E) C4M and (F) VICM were dichotomized into quartiles (Q4, representing the highest levels of the biomarker). P values are based on the log-rank test. C3M, C4M, matrix metalloprotease (MMP)-degraded type III and IV collagen; C4G, granzyme B-degraded type IV collagen; PC3X, cross-linked N-terminal pro-peptide of type III collagen; PRO-C3, N-terminal pro-peptide of type III collagen; VICM, citrullinated and MMP-degraded vimentin.
Figure 2.
Association of extracellular matrix (ECM) biomarkers measured at baseline and overall survival (OS). Kaplan-Meier plots demonstrating the association of ECM biomarkers with OS for patients with metastatic melanoma. (A) PRO-C3, (B) PC3X, (C) C4G, (D) C3M, (E) C4M and (F) VICM were dichotomized into quartiles (Q4, representing the highest levels of the biomarker). P values are based on the log-rank test. C3M, C4M, matrix metalloprotease (MMP)-degraded type III and IV collagen; C4G, granzyme B-degraded type IV collagen; PC3X, cross-linked N-terminal pro-peptide of type III collagen; PRO-C3, N-terminal pro-peptide of type III collagen; VICM, citrullinated and MMP-degraded vimentin.
Figure 3.
A high C3M/PRO-C3 ratio at baseline associate with good survival outcomes. Kaplan-Meier plots demonstrating the association of the C3M/PRO-C3 ratio with progression-free survival (PFS) (A) and overall survival (OS) (B) for patients with metastatic melanoma. Patients were divided based on a C3M/PRO-C3 ratio >1 or <1 and a log-rank test was used to determine differences between survival curves. C3M, matrix metalloprotease (MMP)-degraded type III collagen; PRO-C3, N-terminal pro-peptide of type III collagen.
jitc-2020-001193supp001.pdf (237.3KB, pdf)
Excessive type III collagen turnover are independently related to worse outcome when assessed by Cox regression analysis
To evaluate association of the biomarkers with PFS and OS, univariate Cox regression analyses were performed of all ECM biomarkers and the common baseline patient risk factors: gender, age, WHO, brain metastasis and LDH.
When assessing PFS, then LDH, PRO-C3, PC3X, C4G and C3M levels as continuous test variables were associated with worse PFS (table 2).
Table 2.
Univariate and multivariate Cox regression analysis of the association of patient factors and ECM biomarkers with progression-free survival (PFS)
| Factor | Test variable | Events/total cases | Univariate | P value | Events/Total cases |
Multivariate Adjusted for LDH |
P value |
| HR (95% CI) | HR (95% CI) | ||||||
| Gender | Male vs female | 40/62 vs 26/44 | 1.14 (0.69 to 1.86) | 0.611 | |||
| Age | Continuous (year) | 66/106 | 1.00 (0.99 to 1.02) | 0.744 | |||
| WHO | >1 vs 0 | 27/37 vs 39/69 | 1.60 (0.98 to 2.62) | 0.061 | |||
| Brain metastasis | Present vs absent | 12/18 vs 33/47 | 1.00 (0.52 to 1.94) | 0.997 | |||
| LDH | Continuous (U/L) | 66/106 | 1.00 (1.00 to 1.00) | 0.014* | |||
| PRO-C3 | Continuous (ng/mL) | 66/106 | 1.02 (1.00 to 1.04) | 0.031* | |||
| 4.0–12.5 ng/mL, Q1-–Q3 | 45/79 | 1.00 | – | 45/79 | |||
| 12.6–84.7 ng/mL, Q4 | 21/27 | 1.96 (1.17 to 3.30) | 0.011* | 21/27 | 1.81 (1.06 to 3.10) | 0.030* | |
| PC3X | Continuous (ng/mL) | 66/10644/79 | 1.04 (1.00 to 1.07) | 0.046* | |||
| 1.2–7.6 ng/mL, Q1-–Q3 | 1.00 | – | 44/79 | ||||
| 7.6–30.2 ng/mL, Q4 | 22/27 | 2.05 (1.23 to 3.43) | 0.006** | 22/27 | 1.86 (1.09 to 3.18) | 0.023* | |
| C4G | Continuous (ng/mL) | 65/105 | 1.01 (1.00 to 1.02) | 0.019* | |||
| 7.8–27.3 ng/mL, Q1-–Q3 | 44/79 | 1.00 | – | 44/79 | |||
| 27.4–125.7 ng/mL, Q4 | 21/26 | 1.93 (1.15 to 3.26) | 0.014* | 21/26 | 1.91 (1.13 to 3.23) | 0.016* | |
| C3M | Continuous (ng/mL) | 66/106 | 1.04 (1.00 to 1.09) | 0.038* | |||
| 8.2–19.5 ng/mL, Q1–Q3 | 45/79 | 1.00 | – | 45/79 | |||
| 19.6–36.0 ng/mL, Q4 | 21/27 | 1.75 (1.04 to 2.93) | 0.036* | 21/27 | 1.67 (0.99 to 2.82) | 0.054 | |
| C4M | Continuous (ng/mL) | 66/106 | 1.01 (1.00 to 1.03) | 0.158 | |||
| 8.8–38.0 ng/mL, Q1–Q3 | 47/79 | 1.00 | – | 47/79 | |||
| 38.8–87.1 ng/mL, Q4 | 19/27 | 1.29 (0.76 to 2.20) | 0.349 | 19/27 | 1.19 (0.69 to 2.04) | 0.532 | |
| VICM | Continuous (ng/mL) | 66/106 | 1.01 (1.00 to 1.02) | 0.121 | |||
| 1.0–23.4 ng/mL, Q1–Q3 | 48/79 | 1.00 | – | 48/79 | |||
| 23.7–132.3 ng/mL, Q4 | 18/27 | 1.16 (0.67 to 1.99) | 0.602 | 18/27 | 1.27 (0.73 to 2.20) | 0.401 | |
| C3M/PRO-C3 | >1 vs <1 | 51/88 vs 15/18 | 0.51 (0.29 to 0.91) | 0.023* | 51/88 vs 15/18 | 0.59 (0.32 to 1.11) | 0.102 |
HR were calculated by univariate and multivariate cox regression analysis. By the multivariate analysis, the individual biomarkers were adjusted for LDH. Significance is marked with stars.
C4G, granzyme B-degraded type IV collagen; C3M, C4M, matrix metalloprotease (MMP)-degraded type III and IV collagen; ECM, extracellular matrix; LDH, lactate dehydrogenase; PC3X, cross-linked N-terminal pro-peptide of type III collagen; PRO-C3, N-terminal pro-peptide of type III collagen; VICM, citrullinated and MMP-degraded vimentin; WHO, WHO performance score.
Based on the Kaplan-Meier plot, the predictive value was also evaluated by using the 75th percentile cut-point (Q4) for the ECM biomarkers. Here, higher baseline levels (Q4) of PRO-C3 (HR=1.96, 95% CI=1.17 to 3.30, p=0.011), PC3X (HR=2.05, 95% CI=1.23 to 3.43, p=0.006), C4G (HR=1.93, 95% CI=1.15 to 3.26, p=0.014) and C3M (HR=1.75, 95% CI=1.04 to 2.93, p=0.036) were negatively related to PFS compared with lower levels (Q1–Q3) (table 2). Moreover, a high C3M/PRO-C3 ratio (>1) was associated with improved PFS (HR=0.51, 95% CI=0.29 to 0.91, p=0.023).
In the multivariate analysis, PRO-C3 and PC3X levels were both independently related to worse PFS after correction for LDH (HR=1.81, 95% CI=1.06 to 3.10, p=0.030 and HR=1.86, 95% CI=1.09 to 3.18, p=0.023, respectively; table 2), although baseline PRO-C3 and PC3X were moderately correlated with baseline LDH (p<0.0001; online supplemental table 1).
When assessing OS in the univariate analysis, PRO-C3, PC3X and LDH as continuous variables and brain metastases and WHO as categorical variables were negatively associated with OS (table 3). Similar to PFS, high PRO-C3 (Q4) (HR=2.41, 95% CI=1.26 to 4.60, p=0.008) and PC3X (HR=2.21, 95% CI=1.14 to 4.28, p=0.019) were associated with worse OS compared with lower levels (Q1–Q3) (table 3). Again, a high C3M/PRO-C3 ratio (>1) was related to improved OS (HR=0.33, 95% CI=0.16 to 0.65, p=0.001) compared with a low ratio (table 3).
Table 3.
Univariate and multivariate Cox regression analysis of the association of patient factors and ECM biomarkers with overall survival (OS)
| Factor | Test variable | Events/Total cases | Univariate | P value | Events/Total cases | Multivariate Adjusted for LDH |
P value | Events/Total cases | Multivariate Adjusted for ¤ |
P value |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||||
| Gender | Male vs female | 25/62 vs 15/45 | 1.31 (0.68 to 2.51) | 0.426 | ||||||
| Age | Continuous (years) | 40/107 | 1.01 (0.99 to 1.03) | 0.410 | ||||||
| WHO | >1 vs 0 | 23/38 vs 17/69 vs | 3.10 (1.64 to 5.86) | <0.001*** | ||||||
| Brain metastases | Present vs absent | 9/18 vs 13/48 | 2.39 (1.01 to 5.68) | 0.049* | ||||||
| LDH | Continuous (U/L) | 40/107 | 1.00 (1.00 to 1.00) | <0.0001**** | ||||||
| PRO-C3 | Continuous (ng/mL) | 40/107 | 1.03 (1.01 to 1.05) | 0.004** | ||||||
| 4.0–12.6 ng/mL, Q1–Q3 | 25/80 | 1.00 | – | 25/80 | 14/49 | |||||
| 12.7–84.7 ng/mL, Q4 | 15/27 | 2.41 (1.26 to 4.60) | 0.008** | 15/27 | 2.08 (1.05 to 4.09) | 0.035* | 8/17 | 1.87 (0.71 to 4.90) | 0.202 | |
| PC3X | Continuous (ng/mL) | 40/107 | 1.04 (1.00 to 1.09) | 0.045* | ||||||
| 1.2–7.6 ng/mL, Q1–Q3 | 26/80 | 1.00 | – | 26/80 | 16/50 | |||||
| 7.6–30.2 ng/mL, Q4 | 14/27 | 2.21 (1.14 to 4.28) | 0.019* | 14/27 | 1.78 (0.88 to 3.60) | 0.106 | 6/16 | 1.03 (0.38 to 2.75) | 0.954 | |
| C4G | Continuous (ng/mL) | 39/106 | 1.00 (0.98 to 1.02) | 0.693 | ||||||
| 7.8–27.3 ng/mL, Q1–Q3 | 31/80 | 1.00 | – | 31/80 | 15/46 | |||||
| 27.4–125.7 ng/mL, Q4 | 8/26 | 0.93 (0.42 to 2.04) | 0.856 | 8/26 | 0.95 (0.43 to 2.09) | 0.902 | 6/19 | 1.68 (0.63 to 4.53) | 0.302 | |
| C3M | Continuous (ng/mL) | 40/107 | 1.02 (0.96 to 1.08) | 0.484 | ||||||
| 8.2–19.5 ng/mL, Q1–Q3 | 29/80 | 1.00 | – | 29/80 | 17/49 | |||||
| 19.6–36.0 ng/mL, Q4 | 11/27 | 1.12 (0.54 to 2.30) | 0.760 | 11/27 | 1.10 (0.54 to 2.27) | 0.788 | 5/17 | 0.71 (0.24 to 2.14) | 0.544 | |
| C4M | Continuous (ng/mL) | 40/107 | 1.01 (0.99 to 1.04) | 0.237 | ||||||
| 8.8–38.0 ng/mL, Q1–Q3 | 29/80 | 1.00 | – | 29/80 | 18/51 | |||||
| 38.8–87.1 ng/mL, Q4 | 11/27 | 1.27 (0.63 to 2.56) | 0.507 | 11/27 | 1.14 (0.56 to 2.30) | 0.720 | 4/15 | 0.66 (0.22 to 1.98) | 0.458 | |
| VICM | Continuous (ng/mL) | 40/107 | 1.01 (0.99 to 1.02) | 0.344 | ||||||
| 1.0–23.4 ng/mL, Q1–Q3 | 30/79 | 1.00 | – | 30/79 | 15/48 | |||||
| 23.7–132.3 ng/mL, Q4 | 10/28 | 0.97 (0.47 to 1.98) | 0.926 | 10/28 | 1.14 (0.55 to 2.37) | 0.731 | 7/18 | 1.23 (0.48 to 3.11) | 0.666 | |
| C3M/PRO-C3 | >1 vs <1 | 28/88 vs 12/19 | 0.33 (0.16 to 0.65) | 0.001* | 28/88 vs 12/19 | 0.42 (0.20 to 0.90) | 0.025* | 15/55 vs 7/11 | 0.39 (0.14 to 1.06) | 0.066 |
HR were calculated by univariate and multivariate cox regression analysis. In one multivariate analysis, the individual biomarkers were adjusted for LDH and in the other they were adjusted for LDH, WHO and brain metastases (indicated by ¤). Significance is marked with stars.
C4G, granzyme B-degraded type IV collagen; C3M, C4M, matrix metalloprotease (MMP)-degraded type III and IV collagen; ECM, extracellular matrix; LDH, lactate dehydrogenase; PC3X, cross-linked N-terminal pro-peptide of type III collagen; PRO-C3, N-terminal pro-peptide of type III collagen; VICM, citrullinated and MMP-degraded vimentin; WHO, WHO performance score.
In the multivariate analysis, two models were tested. In the first model, each ECM biomarker was adjusted for the commonly used serum marker LDH. In the second model, the biomarkers were adjusted for both LDH, WHO and brain metastases as all three factors were predictive for OS. Two models were tested because information about brain metastases is missing in about 40% of cases.
Importantly, PRO-C3 remained significantly associated with worse OS after adjustment for LDH (HR=2.08, 95% CI=1.06 to 4.09, p=0.035), with a similar trend for PC3X that did not however meet statistical significance (HR=1.78, 95% CI=0.88 to 3.60, p=0.106; table 3). In line with previous findings, a high C3M/PRO-C3 ratio was related to improved OS when adjusted for LDH (HR=0.42, 95% CI=0.20 to 0.90, p=0.025; table 3). When all three factors (LDH, WHO and brain metastases) were included in the multivariate analysis, the predictive values were attenuated.
When assessing the effects of nivolumab versus pembrolizumab on PFS and OS in patients with low (Q1–Q3) and high (Q4) biomarker levels, respectively, the only significant association was found for patients with low PC3X, who had improved PFS when treated with pembrolizumab compared with nivolumab (HR=0.51, 95% CI=0.27 to 0.96, p=0.038) (online supplemental table 2).
Baseline levels of PRO-C3 are associated with progressive disease
The relationship between baseline levels of the ECM biomarkers and the BOR was assessed. When comparing patients with PD with patients achieving disease control (BOR of SD, PR or CR), baseline PRO-C3 levels were significantly elevated in patients with PD (median 10.5 ng/mL; 95% CI=8.3 to 12.7 vs 8.8 ng/mL; 95% CI=7.6 to 9.9, p=0.046; online supplemental figure 2A). No significant differences were observed for the remaining five biomarkers (online supplemental figure 2B–F).
jitc-2020-001193supp003.pdf (392.6KB, pdf)
An increase of VICM levels during treatment was positively associated with clinical outcome
The dynamics of collagen and VICM turnover were assessed during PD-1 inhibition by comparing the baseline biomarker levels with the levels after PD-1 ICI therapy. For PRO-C3, PC3X and VICM, an increase of serum levels was observed after 6 weeks of therapy compared with baseline (PRO-C3: 10.2 ng/mL; 95% CI=9.4 to 12.1 vs 9.0 ng/mL; 95% CI=8.3 to 9.9; p<0.0001, PC3X: 5.7 ng/mL; 95% CI=5.2 to 6.3 vs 4.9 ng/mL; 95% CI=4.4 to 5.6; p=0.003 and VICM: 21.4 ng/mL; 95% CI=17.0 to 25.7 vs 15.0 ng/mL 95% CI=12.6 to 18.1; p<0.0001; online supplemental figure 3). Neither C4G, C3M or C4M changed significantly after treatment. Of note, the levels of all six biomarkers were highly correlated before and after 6 weeks of treatment (online supplemental table 3). Next, the association between biomarker levels during therapy and PFS and OS was assessed.
jitc-2020-001193supp004.pdf (476.6KB, pdf)
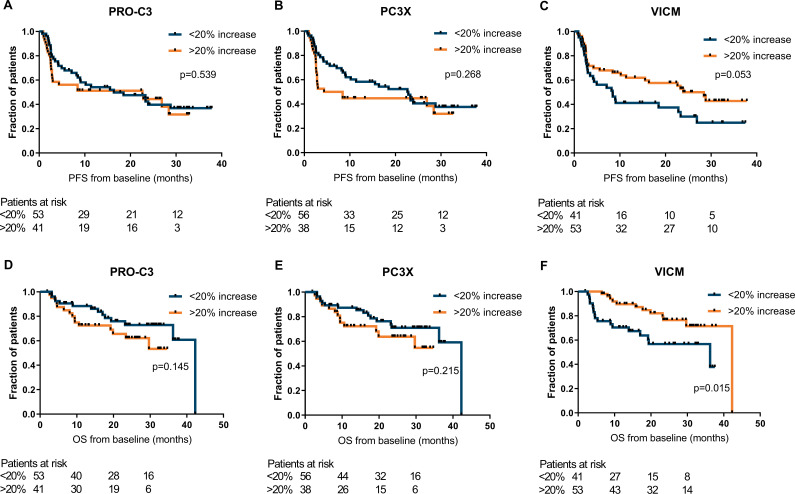
By the Kaplan-Meier approach, moderate significant differences of PFS were observed for quartile groups of PRO-C3 (p=0.011), C4G (p=0.017) and C4M (p=0.046) levels (online supplemental figure 4). The levels of the ECM biomarkers during therapy were not significantly associated with OS (online supplemental figure 5). Next, stratification was performed into patients who had an evident increase of the ECM biomarker during therapy (of >20% from baseline) and patients who did not have an evident increase (the remaining patients). Patients with an increase of VICM during therapy seemed to have, although not significant, an improved PFS assessed by the Kaplan-Meier approach (median PFS: 28.4 vs 8.4 months, p=0.053) (figure 4C) and by univariate Cox regression (HR=0.59, 95% CI=0.35 to 1.01, p=0.056). The OS in these patients was significantly improved (Kaplan-Meier (figure 4F): median OS: 42.3 vs 36.2 months, p=0.015 and univariate Cox regression: HR=0.39, 95% CI=0.19 to 0.86, p=0.018). VICM remained significant after correction for WHO performance status, brain metastases and LDH (HR=0.28, 95% CI=0.10 to 0.77, p=0.014) and LDH alone (HR=0.42, 95% CI=0.20 to 0.91, p=0.028). No significant differences were observed for PRO-C3 or PC3X, although a trend could be observed for increased PRO-C3 and PC3X levels and worse OS (figure 4A–E).
Figure 4.
Increase in VICM after anti-programmed cell death protein 1 (anti-PD-1) treatment associates with progression-free survival (PFS) and overall survival (OS). Kaplan-Meier plots demonstrating the association of change in PRO-C3 (A, D), PC3X (B, E) and VICM (C, F) with PFS and OS 6 weeks after start of anti-PD-1 immune checkpoint inhibitor (ICI) therapy after stratification of patients based on an increase of >20% from baseline vs <20% increase. P values are based on the log-rank test. PC3X, cross-linked N-terminal pro-peptide of type III collagen; PRO-C3, N-terminal pro-peptide of type III collagen; VICM, citrullinated and MMP-degraded vimentin.
jitc-2020-001193supp005.pdf (834.3KB, pdf)
jitc-2020-001193supp006.pdf (859.3KB, pdf)
Discussion
The present study demonstrates that blood-based biomarkers of protease-generated neo-epitopes on protein fragments reflecting altered ECM turnover are associated with clinical outcome after PD-1 ICI therapy in metastatic melanoma. Baseline serum levels of ECM protein fragments PRO-C3 and PC3X, indicating a collagen-rich peritumoral stroma and fibrotic activity, were associated with resistance to PD-1 inhibition. Moreover, upregulation of VICM during therapy, reflecting M1 macrophage activity, was associated with improved survival.
Patients with higher PRO-C3 and PC3X levels had worse clinical outcome after PD-1 inhibition compared with patients with lower levels. Interestingly, as PRO-C3 and PC3X are released to the blood circulation during excessive collagen formation and cross-linking,16 the findings suggest that those markers identify tumors with an immune-excluded phenotype. Immune-exclusion is characterized by dense collagen stroma that limits the entry of tumor infiltrating lymphocytes into the TME, which is considered as an important biological mechanism of therapy resistance.4 17 Cytotoxic T cell activity towards cancer cells in the TME is suggested to be an independent factor of the PD-L1 tumor proportion score and tumor mutation burden.2 18
Although these protease-generated protein fragments were significantly correlated to LDH levels, we demonstrated that higher PRO-C3, independent of LDH, was associated with worse PFS and OS and that higher PC3X was associated with worse PFS. LDH is known as a marker of tumor burden and a clinically significant adverse prognostic factor for metastatic melanoma, which is also confirmed in this immuno-oncology setting.19 Elevated LDH levels is integrated as a suffix to the M1 subcategories in recent TNM cancer staging protocols,20 providing additional prognostic value. Interestingly, similar observations for PRO-C3 were previously seen in patients with metastatic melanoma who were treated with CTLA-4 ICI therapy,13 suggesting that the non-invasive biomarker may be considered across different ICIs.
Dense collagen stroma may also affect the diffusion and distribution of the therapeutic antibodies,21 in addition to its effect in limiting the entry of tumor infiltrating lymphocytes into the TME. Previous work by Jensen et al13 demonstrated additional prognostic value of the ratio of type III collagen degradation to formation (C3M/PRO-C3) after CTLA-4 inhibition in metastatic melanoma. The present findings confirm that patients with a high C3M/PRO-C3 ratio (>1) are linked to better clinical outcome after PD-1 inhibition, thereby providing additional evidence for the hypothesis that patients with relatively low net fibrosis/collagen deposition are more likely to respond to ICIs. PRO-C3 and PC3X are associated with a higher activity of CAFs. In addition to the desmoplastic, collagen-rich TME related to immune-exclusion, CAFs have also shown to contribute to a diminished immune function by expressing TGF-β, PD-L1/PD-L2 and by inducing immune checkpoints on T cells.6 22–24
We demonstrated that patients with an increase in VICM during treatment had improved clinical outcome. VICM is released from activated inflammatory M1 macrophages,25 which may be an important factor for response to ICIs compared with the immune-suppressive M2 macrophage phenotype. For example, PD-L1 inhibition was demonstrated to polarize macrophages into a more pro-inflammatory phenotype, which was associated with enhanced T cell activity.26 In mice, PD-1 inhibition was found to increase macrophage phagocytosis, and was associated with better survival in a macrophage-dependent fashion.27 Our results support these findings and additionally suggest that blood-based VICM levels have potential value for monitoring early response to PD-1 inhibition.
Whereas higher C4M levels at baseline were previously demonstrated to be associated with worse clinical outcome after CTLA-4 inhibition in metastatic melanoma,13 we could not confirm this observation after PD-1 inhibition. C4M mirrors basement membrane remodeling, which is strongly associated with malignant progression of cancer.28 These inconsistent results may be explained either by the difference of mechanism of action of CTLA-4 and PD-1 inhibition or by the higher efficacy of PD-1 inhibition in this immuno-oncology setting.13
Interestingly, C4G levels were previously associated with improved OS after CTLA-4 inhibition in metastatic melanoma.29 This C4G biomarker measures a neo-epitope of C4G that may reflect cytotoxic T-cell infiltration in the TME.30 Nevertheless, we could not observe a relationship between C4G levels and clinical outcome after PD-1. Granzyme B is a key serine protease that is secreted by activated T and NK cells to induce apoptosis.31 While it is suggested to contribute to response mechanisms of PD-1 inhibition in metastatic lung cancer, illustrated by an inverse relationship of both granzyme B levels as well as germline variation of GZMB and clinical outcome,32 further research is needed to elaborate on the differences between ICIs and tumor types.33
Despite the interesting findings, this study also has some limitations. Patients were treated with two different ICIs (nivolumab or pembrolizumab) and not designed/powered to differentiate between the two. Although these are highly similar monoclonal antibodies directed to the PD-1 receptor, this may have an impact on the results. Furthermore, the sample size was too small to correct for multiple testing or for splitting the patients into a discovery and validation group. Nevertheless, in the current analysis we were able to demonstrate strong associations with survival outcomes for a selection of ECM biomarkers. Although ECM turnover is a physiological process taking place throughout the body, the assessments of specific post-translational modifications (neo-epitopes) may reduce the systemic background of healthy turnover and instead increase the specificity of ECM turnover in the TME.12 34 Besides, even though our main objective was to investigate the association of markers with clinical outcome, the findings do not address the predictive performance of the biomarkers, for which prospective external validation is needed in cohorts randomized to different modalities. Lastly, a statistical methodology is needed to determine the cut-off discriminating high and low levels of these molecules. This is of particular importance for validation in a randomized setting for establishing the performance of biomarkers for diagnostic use, which was not the scope of this study. Here, cutoffs may be selected by receiver operating characteristics. We believe that identification of patients who have primary resistance should require a high negative predictive value, consequently with stringent cut-off values.
Conclusions
In conclusion, we demonstrated that the ECM protein fragments PRO-C3 and PC3X in baseline serum, representing a dense collagen stroma, were associated with worse clinical outcome after PD-1 inhibition in metastatic melanoma. Moreover, increasing VICM levels early during therapy was associated with improved survival. VICM levels correspond with M1 macrophage activity by its specific release of MMP-degraded and citrullinated vimentin in the blood circulation. After validation, these biomarkers may provide a robust non-invasive tool for patient stratification and therapeutic decision-making in immuno-oncology.
Footnotes
DPH and CJ contributed equally.
Contributors: DPH and CJ contributed to the conception and design of the study. DPH and CJ drafted the manuscript. DPH and CJ contributed to the acquisition, analysis of data and contributed statistical review of data. All authors took part in interpretation of data. DPH and RHJM performed radiological evaluation. CJ performed the ECM biomarker measurements. All authors contributed to critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding: This research was funded by the Departments of Medical Oncology and Pulmonology, Erasmus MC, Rotterdam, The Netherlands and the Danish Research Foundation.
Competing interests: JA reports personal fees from MSD, BMS, Amphera, Eli-Lilly, Takeda, Bayer, Roche, Boehringer Ingelheim, AstraZeneca outside the submitted work, and has a patent allogenic tumor cell lysate licensed to Amphera, a patent combination immunotherapy in cancer and a patent biomarker for immunotherapy pending. RHJM reports grants and non-financial support from Astellas, Bayer and Boehringer Ingelheim, grants from Cristal Therapeutics and Pamgene, grants and personal fees from Novartis, Servier, grants and non-financial support from Pfizer, grants from Roche, Sanofi, outside the submitted work. MAK, NW and CJ are employed at Nordic Bioscience A/S, which is a company involved in discovery and development of biomarkers. MAK owns stocks at Nordic Bioscience.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the independent ethics committee board (reference number: MEC 16–011; Medical Ethical Board Erasmus MC, Rotterdam, The Netherlands) and all patients provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Jessurun CAC, Vos JAM, Limpens J, et al. . Biomarkers for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Front Oncol 2017;7:233. 10.3389/fonc.2017.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurkmans DP, Kuipers ME, Smit J, et al. . Tumor mutational load, CD8+ T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol Immunother 2020;69:771–7. 10.1007/s00262-020-02506-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarhini A, Kudchadkar RR. Predictive and on-treatment monitoring biomarkers in advanced melanoma: moving toward personalized medicine. Cancer Treat Rev 2018;71:8–18. 10.1016/j.ctrv.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 4.Mariathasan S, Turley SJ, Nickles D, et al. . TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–8. 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Saci A, Szabo PM, et al. . EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat Commun 2018;9:3503. 10.1038/s41467-018-05992-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravarthy A, Khan L, Bensler NP, et al. . TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun 2018;9:4692. 10.1038/s41467-018-06654-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okrah K, Tarighat S, Liu B, et al. . Transcriptomic analysis of hepatocellular carcinoma reveals molecular features of disease progression and tumor immune biology. NPJ Precis Oncol 2018;2:25. 10.1038/s41698-018-0068-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mushtaq MU, Papadas A, Pagenkopf A, et al. . Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers. J Immunother Cancer 2018;6:65. 10.1186/s40425-018-0376-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziani L, Safta-Saadoun TB, Gourbeix J, et al. . Melanoma-Associated fibroblasts decrease tumor cell susceptibility to NK cell-mediated killing through matrix-metalloproteinases secretion. Oncotarget 2017;8:19780–94. 10.18632/oncotarget.15540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchenreuther J, Leask A. Why target the tumor stroma in melanoma? J Cell Commun Signal 2018;12:113–8. 10.1007/s12079-017-0419-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52–67. 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leeming DJ, Bay-Jensen AC, Vassiliadis E, et al. . Post-Translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers 2011;16:193–205. 10.3109/1354750X.2011.557440 [DOI] [PubMed] [Google Scholar]
- 13.Jensen C, Madsen DH, Hansen M, et al. . Non-Invasive biomarkers derived from the extracellular matrix associate with response to immune checkpoint blockade (anti-CTLA-4) in metastatic melanoma patients. J Immunother Cancer 2018;6:152. 10.1186/s40425-018-0474-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willumsen N, Ali SM, Leitzel K, et al. . Collagen fragments quantified in serum as measures of desmoplasia associate with survival outcome in patients with advanced pancreatic cancer. Sci Rep 2019;9:19761. 10.1038/s41598-019-56268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16.Nielsen MJ, Nedergaard AF, Sun S, et al. . The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res 2013;5:303–15. [PMC free article] [PubMed] [Google Scholar]
- 17.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–30. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 18.Rizvi NA, Hellmann MD, Snyder A, et al. . Mutational landscape determines sensitivity to PD-1 blockade in non. Science 2016;348:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weide B, Martens A, Hassel JC, et al. . Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 2016;22:5487–96. 10.1158/1078-0432.CCR-16-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershenwald JE, Scolyer RA, Hess KR, et al. . Melanoma staging: evidence-based changes in the American joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:472–92. 10.3322/caac.21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis DM, Thomas SN. Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Adv Drug Deliv Rev 2017;114:33–42. 10.1016/j.addr.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmadzadeh M, Rosenberg SA. TGF-β1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol 2005;174:5215–23. 10.4049/jimmunol.174.9.5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nissen NI, Karsdal M, Willumsen N. Collagens and cancer associated fibroblasts in the reactive stroma and its relation to cancer biology. J Exp Clin Cancer Res 2019;38:115. 10.1186/s13046-019-1110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorchs L, Fernández Moro C, Bankhead P, et al. . Human pancreatic carcinoma-associated fibroblasts promote expression of co-inhibitory markers on CD4+ and CD8+ T-cells. Front Immunol 2019;10:847. 10.3389/fimmu.2019.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortensen JH, Guo X, De Los Reyes M, et al. . The VICM biomarker is released from activated macrophages and inhibited by anti-GM-CSFRα-mAb treatment in rheumatoid arthritis patients. Clin Exp Rheumatol 2019;37:73–80. [PubMed] [Google Scholar]
- 26.Xiong H, Mittman S, Rodriguez R, et al. . Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res 2019;79:1493–506. 10.1158/0008-5472.CAN-18-3208 [DOI] [PubMed] [Google Scholar]
- 27.Gordon SR, Maute RL, Dulken BW, et al. . PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545:495–9. 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 2011;4:165–78. 10.1242/dmm.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen C, Sinkeviciute D, Madsen DH, et al. . Granzyme B degraded type IV collagen products in serum identify melanoma patients responding to immune checkpoint blockade. Cancers 2020;12:2786. 10.3390/cancers12102786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakash MD, Munoz MA, Jain R, et al. . Granzyme B promotes cytotoxic lymphocyte transmigration via basement membrane remodeling. Immunity 2014;41:960–72. 10.1016/j.immuni.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 31.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 2015;15:388–400. 10.1038/nri3839 [DOI] [PubMed] [Google Scholar]
- 32.Hurkmans DP, Basak EA, Schepers N, et al. . Granzyme B is correlated with clinical outcome after PD-1 blockade in patients with stage IV non-small-cell lung cancer. J Immunother Cancer 2020;8:e000586. 10.1136/jitc-2020-000586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei SC, Levine JH, Cogdill AP, et al. . Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 2017;170:1120–33. 10.1016/j.cell.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karsdal MA, Henriksen K, Leeming DJ, et al. . Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers--are they the cause or the consequence of the disease? Clin Biochem 2010;43:793–804. 10.1016/j.clinbiochem.2010.03.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001193supp002.pdf (305.5KB, pdf)
jitc-2020-001193supp001.pdf (237.3KB, pdf)
jitc-2020-001193supp003.pdf (392.6KB, pdf)
jitc-2020-001193supp004.pdf (476.6KB, pdf)
jitc-2020-001193supp005.pdf (834.3KB, pdf)
jitc-2020-001193supp006.pdf (859.3KB, pdf)