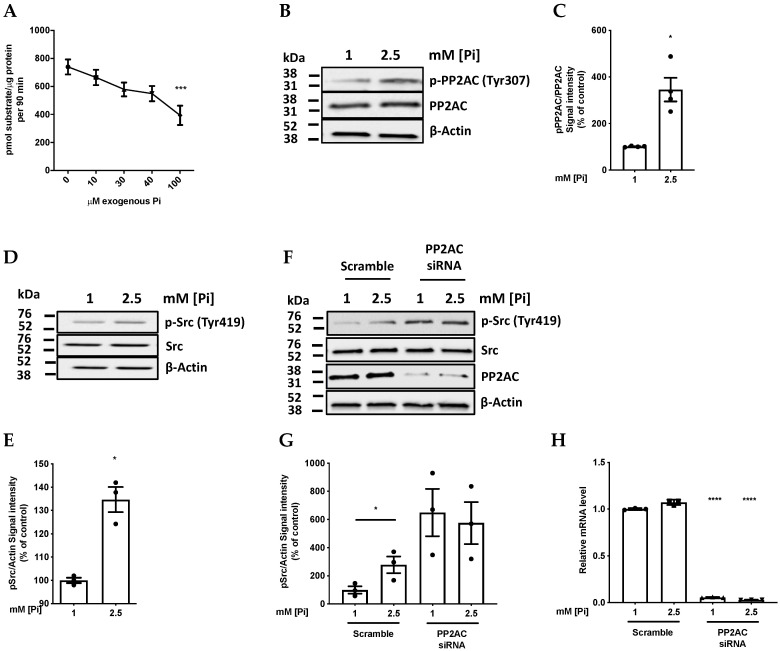
Figure 2.
Pi inhibits PP2A and induces an increase in Src phosphorylation. (A) Direct inhibition by Pi of PP2A catalytic activity after pulling-down endogenous PP2A-C from EA.hy926 ECs lysates and subjecting this to phosphatase catalytic activity assay in the presence of exogenous Pi at the stated concentration using PP2A phosphatase substrate (Millipore Ref 17-313). n = 3, *** p = 0.0002 versus control without Pi. (B) Representative immunoblot demonstrating Pi-induced inhibitory phosphorylation of PP2A in intact EA.hy926 cells. Effect of 1.5 h Pi-loading hyperphosphatemia on expression of PP2AC and p-PP2AC (Tyr307). (C) Densitometry analysis of (B), n = 3, * p = 0.037. (D) Representative p-Src immunoblots probed using anti-p-Src (Tyr419) antibody and (E) corresponding densitometry analysis of (D) n = 3, * p = 0.023. (F) Representative immunoblots and (G) quantitative analysis by densitometry of the effect of siRNA silencing of PP2A-C on Src phosphorylation during 1.5 h incubations of cells with 1 or 2.5 mM Pi. n = 3, * p = 0.0337. (H) Relative mRNA levels of PP2A-C in EA.hy926 cells transfected with scrambled siRNA and PP2A-C silencing siRNA for 1.5 h. After removal of the transfection medium and allowing an additional 24-h recovery period in growth medium, cultures were treated with 1 or 2.5 mM Pi, and RNA was extracted from the cells, reverse transcribed, and subjected to quantitative RT-PCR. n = 3, **** p < 0.0001.