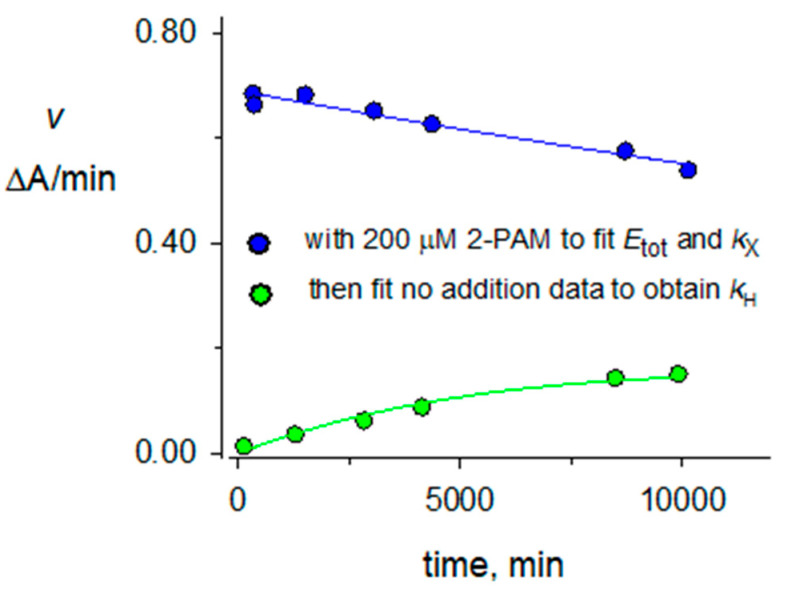
Figure 4.
Denaturation and hydrolysis of diethylphosphorylated AChE in D2O. A mixture of 2.0 μM paraoxon and 1.4 μM AChE in 100 mM sodium phosphate and 0.1% BSA (buffer) in D2O (pH 8.0) was incubated for 70 min at 25 °C. A 4-fold excess of buffer in D2O was added, and 200 μL was applied to a 0.5 mL Sephadex G50 spin column (Fisher) that had been prewashed four times in buffer in D2O and centrifuged at 1000× g for 1 min. The eluent was cooled to 4 °C, and 60 μL was added either to 640 μL of buffer in D2O at 25 °C or 640 μL of the same buffer with 2-PAM (to 200 μM) at 25 °C. Aliquots (30 μL) were assayed as in Figure 3 at the times indicated on the x-axis. Assay values v for the solution containing 2-PAM (blue points that correspond to enzyme denaturation) were first fit to Equation (2) with taged set to 0 and fixed initial estimates (min−1 for k values) of kA = 4 × 10−5, kH = 4 × 10−5, kr = 2 × 10−2 and E0 = 0.01 ΔA/min to obtain kX = 2.2 × 10−5 and Etot =0.69 ΔA/min. These values of taged, kA, and Etot along with kX = 4 × 10−5 and kr = 0 were then fixed into Equation (2) to fit the assay values v for the solution without 2-PAM (green points that reflect enzyme denaturation and diethylphosphorylated enzyme hydrolysis). This fitting gave E0 = 0.003 ΔA/min and a kH value of (47 + 3) × 10−6 min−1 included in Table 1.