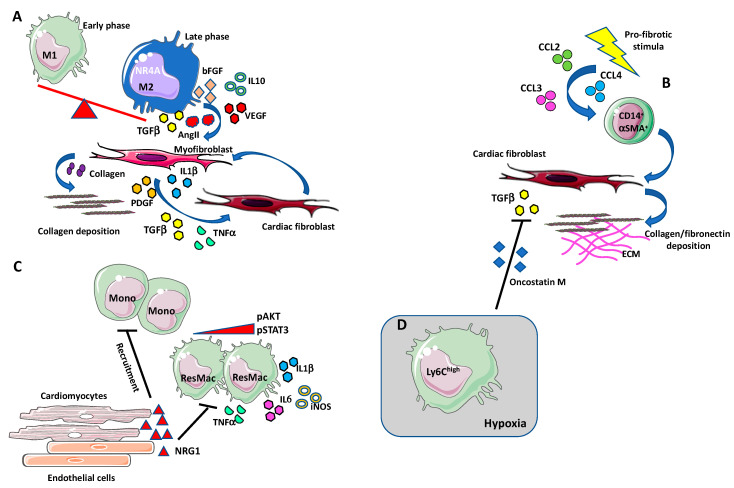
Figure 2.
Macrophages in cardiac fibrosis. (A) During the first phase of tissue inflammation, macrophages acquire a “classically activated” (M1-like) state. In the later phase, macrophages switch into a reparative phenotype, producing anti-inflammatory cytokines, chemokines, and growth factors such as IL-10, TGF-β, VEGF, angiotensin II, bFGF, and PDGF [114,115]. The transition to this reparative state seems to be induced via nuclear receptor subfamily 4 group A member 1 (NR4A1). M2-like macrophages also produce the pro-fibrotic agent TGF-β that induce collagen secreting and collagen stabilizing myofibroblasts. High numbers of macrophages accumulate in the damaged heart, localizing in proximity to myofibroblasts, that, by producing TGF-β, angiotensin II, PDGF, TNFα, and IL-1β, stimulate induce the differentiation of cardiac fibroblasts into myofibroblasts in an autocrine manner. (B) Some of the cardiac infiltrating fibroblast can originate from a circulating monocytic CD14+ cell subset, termed fibrocytes. Under profibrotic stimulation, these cells have been shown to increase the expression of ECM components, such as collagen and fibronectin, and of the mature myofibroblast marker α-SMA. Increased number of circulating fibrocyte has been observed during cardiac fibrosis, in response to the augmented circulating levels of MCP-1/CCL2, CCL4, and CCL3. (C) Neoregulin-1 (NRG-1) can exert antifibrotic and anti-inflammatory effects acting on macrophages in an ErbB4-mediated manner. After fibrotic stimuli, NRG-1, released from damaged endothelial cells in the endocardium, activates ErB4 and downregulates the PI3K/Akt pathway and the phosphorylation of STAT3 thus reducing the release of proinflammatory mediators such as IL-1β, iNOS, IL-6, and TNF-α. The activation of ErbB4 results in the reduction of new monocytes recruitment and suppression of the inflammatory state. (D) Ly6Chigh macrophages infiltrate in hypoxic areas in a hypoxia-inducible factor (HIF-1α)-dependent manner and inhibits TGF-β cardiac fibroblast activation by the release of oncostatin M (OSM).