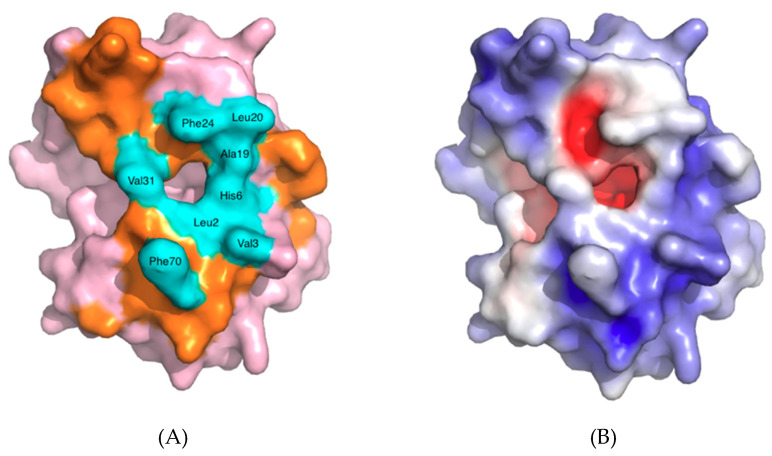
Figure 4.
hGIIA shown with space-filling surface: (A) The amino acids highlighted in cyan, and identified, form the entrance to the active site, and play a role in interfacial binding by making direct contact with the substrate. The interfacial binding surface (i-face) of hGIIA outside of the amino acids at the entrance to the active site are highlighted in orange; (B) Depiction of the electrostatic charge. The high basicity of the protein at the surface is shown by the dominance of blue (positive), over the red of acidity (negative). Confirmation of the non-polar, hydrophobic entrance in orange in the left image is the coincident white of neutrality. The dominance of positive charge is clear. The view 180° to this (not shown) has less negative charge again, but does clearly have both a localised hydrophobic patch at the top in the current view and a localised very positive patch relative to the surface shown at the left hand side region near the C-terminus (behind the right hand side in the views given). Figure used PDB ID 3U8B [13] and created with PyMOL using the Adaptive Poisson-Boltzman Solver for the electrostatics [14]. The orientation is the same as used for Figure 2.