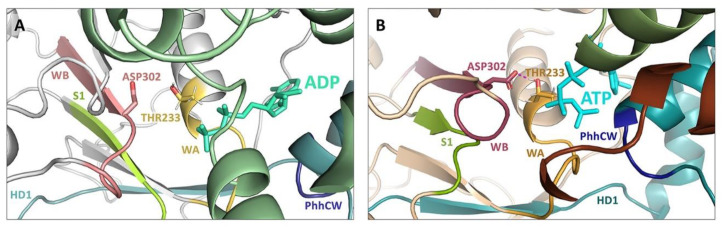
Figure 3.
Molecular dynamics simulations of NLRP3 indicate key residues of the ATP-hydrolysing Walker B motif interact with ATP but not ADP. A critical hydrogen bond between the NLRP3 Walker A (WA) and Walker B (WB) positions the two motifs suitably for hydrolysis of the bound ATP (Thr233 and Asp302, respectively). In (A) the 3.8 Å cryo-EM structure of NLRP3 (PDB: 6NPY) is ADP-bound, and the residues are at a distance of 4.8 Å, exceeding the limit for hydrogen bond activity. However, as shown by a representative snapshot in (B) a 10 ns molecular dynamics simulation of NLRP3 with ATP yielded an average distance of 1.75 Å between the side chain Thr hydroxyl group in WA and Asp carboxyl group in WB, consistent with the formation of a hydrogen bond.