Figure 2:
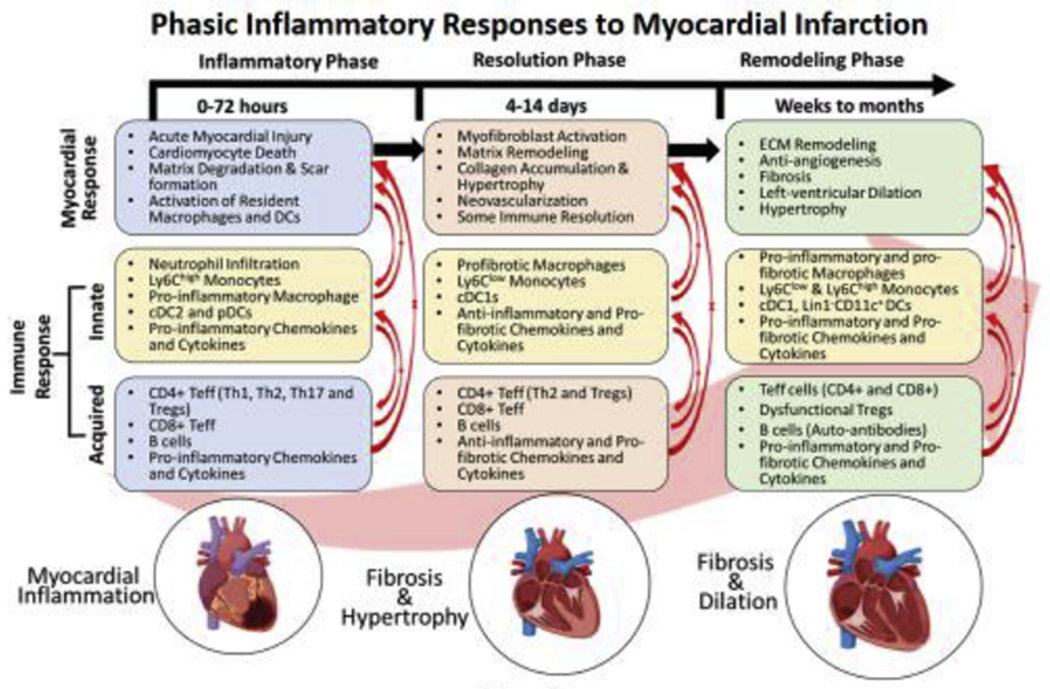
Different phases of immune activation post-MI are temporally regulated to induce intense pro-inflammation from 0-72 h post-injury to clear the damaged cells followed by scar-formation and somewhat immune-resolution from 4-14 days. While the first phase is predominated by the phagocytic and pro-inflammatory cells, 2nd phase is dictated by anti-inflammatory and pro-fibrotic cells. Phase-1 specific chemokines are CXCR2/KC, CXCR4/SDF1α, CCR1/MIP1α, CCR2/MCP1, CXCL1, CXCL12, CXCL13, and CXCL9 whereas cytokines are TNFα, IFNγ, IL-6, and IL-1β. In contrast, phase-II specific chemokines are CCR2/MCP1, CXCR2/CXCR4/MIF, CCR1/MIP1α, CCR5/RANTES, CX3CR1/Fractalkine, CXCL2, CXCL5, CXCL8, and CXCL12, and cytokines are IL-10, TGFβ, CTGF, IL-4, IL-13, IL-6 and eotaxin. Ischemic cardiomyopathy (IC) is associated with low-grade inflammation that over a period of months to years (post-MI) lead to extensive extracellular matrix remodeling, inhibit angiogenesis, and promote LV-remodeling. Both pro- and anti-inflammatory immune responses have been found to be active during this phase suggesting disparate local networks of functionally-similar immune cells. This phase is also associated with several chemokines (CX3CR1/Fractalkine, CXCR4/SDF1α, CCR2/MCP1, CXCL1, CXCL2, CXCL5, and CXCL8) and cytokines (TNFα, IFNγ, TGFβ, IL-10, IL-12, IL-6, and eotaxin). Some of the components were designed using ‘Biorender’.
