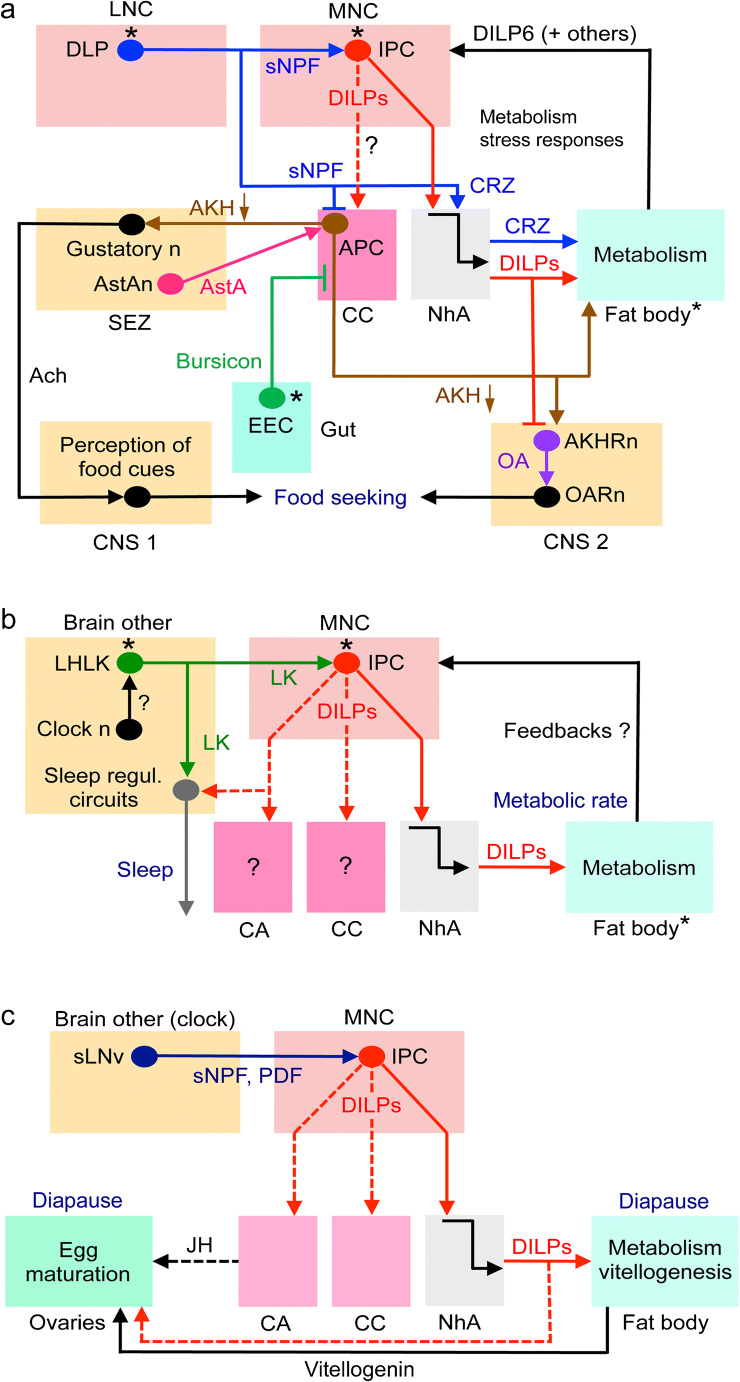
Fig. 5.
Schemes depicting hormonal axes involving insulin-producing cells (IPCs) in adult flies. Note that in this and the following figures with schematic circuits/axes, only one cell of each type is depicted for simplicity. a Adult DLP (LNC) pathway with sNPF and corazonin (CRZ) and regulation of glucose homeostasis, food search and stress responses via IPCs and AKH-producing cells (APCs). Asterisks indicate neurons/cells that are cell autonomously nutrient sensing. The DLPs in the LNC group produce CRZ and sNPF and supply axon terminations to IPCs and APCs. The DLP-derived sNPF regulates IPCs and APCs in corpora cardiaca (CC) and thereby affects glucose homeostasis and metabolic stress responses (Kapan et al. 2012; Oh et al. 2019), whereas CRZ is released into the circulation from the neurohemal area (NhA) associated with CC, the foregut and anterior aorta (Kubrak et al. 2016). CRZ acts on the fat body to regulate metabolic stress and homeostasis (Kubrak et al. 2016). sNPF activates IPCs to increase DILP release and inhibits the APCs to decrease AKH release and thereby affects carbohydrate homeostasis (Kapan et al. 2012; Oh et al. 2019). The signaling from IPCs to APCs in CC (dashed arrow) specifically in response to sNPF has not been demonstrated but DILPs do act on APCs, at least in larvae (Bader et al. 2013). AKH is also known to regulate the sensitivity of AKH receptor–expressing sweet-sensing gustatory neurons that mediate sweet taste (Bharucha et al. 2008; Jourjine et al. 2016). Note that it is not clear whether AKH acts on gustatory neuron processes within the SEZ or their cell body/dendrites in the periphery. Enteroendocrine cells (EECs) of the midgut release bursicon that indirectly inhibits APCs in CC resulting in decreased release of AKH and thus affect glucose homeostasis (Scopelliti et al. 2014). Another regulator of IPCs and APCs is allatostatin A (AstA) but the neuronal pathway mediating this has not been clearly shown (Hentze et al. 2015). Thus, it might be that instead of SEZ neurons, AstA is derived from gut EECs. The fat body may feed back to IPCs by means of DILP6 and other factors such as Upd2, adiponectin and CCHa2 (see Fig. 6, Ahmad et al. 2019; Nässel and Zandawala 2019). Finally, DILPs and AKH regulate octopamine (OA)-producing neurons (AKHRn) that express AKH and DILP receptors. These AKHRn in turn act on octopamine receptor–expressing neurons (OARn) to activate locomotion (Yu et al. 2016). Thus, AKH regulates sensitivity of taste neurons and activates locomotion to increase food search. AKH action also encompasses regulation of activity/rest, depending on time of day (Pauls et al. 2020). Ach, acetylcholine; EEC, enteroendocrine cell; b the IPCs are regulated by leucokinin (LK)-producing neurons, LHLK, in the lateral horn of the brain. The LHLKs, which are under influence of clock neurons (Cavey et al. 2016), also mediate starvation-dependent changes in sleep (Yurgel et al. 2019). The nutrient-sensing LHLKs are part of an LK system in the brain and ventral nerve cord that also regulates physiological processes such as diuresis, metabolism, and organismal activity (Zandawala et al. 2018b). c Activation of IPCs blocks reproductive diapause (i.e., blocks ovarian arrest). The clock neurons, sLNv, use sNPF and PDF to activate the IPCs, which leads to inhibition of diapause, likely due to DILP-mediated activation of vitellogenesis in fat body and egg maturation in ovaries (Nagy et al. 2019b). This probably also involves DILP stimulation of corpora allata (CA) and production of juvenile hormone (JH). Inputs to IPCs from another set of clock neurons (DN1s; not shown here) were shown in another study to regulate feeding rhythm and metabolism (Barber et al. 2016)