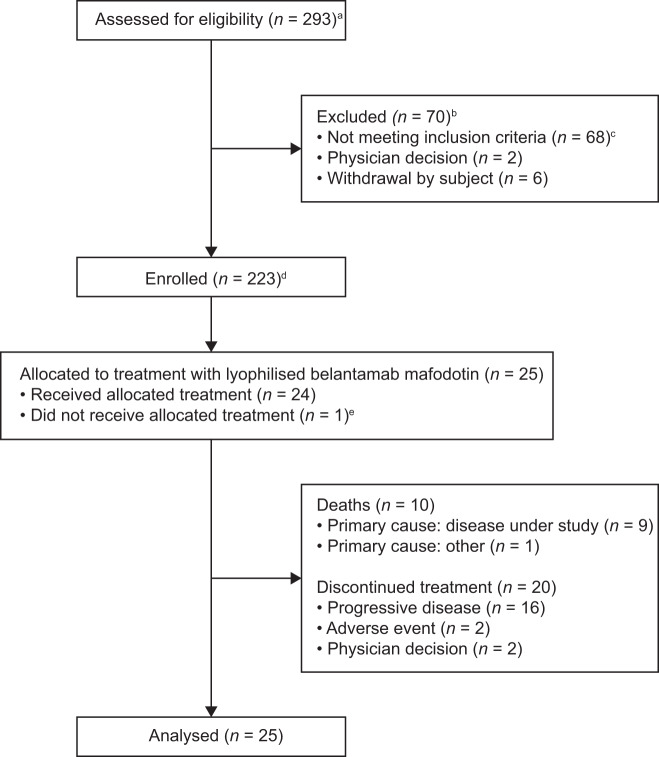
Fig. 1. Patient disposition.
a Between June 2018 and January 2019, 293 patients were screened for inclusion in the entire DREAMM-2 study. Between 5 December 2018 and 10 January 2019, 31 patients were screened for inclusion in the lyophilised presentation cohort. b Patients could have more than one reason for exclusion. c Five patients were excluded due to pre-existing corneal disease, as specified in the study protocol. d The remainder of enrolled patients were included in the main DREAMM-2 study previously reported16. Two patients in the main study were re-randomised and counted twice (once per each randomisation). e One patient was randomised to the belamaf 3.4 mg/kg lyophilised presentation, but actually received 3.4 mg/kg frozen-liquid presentation as first dose, and never received lyophilised presentation during the study.