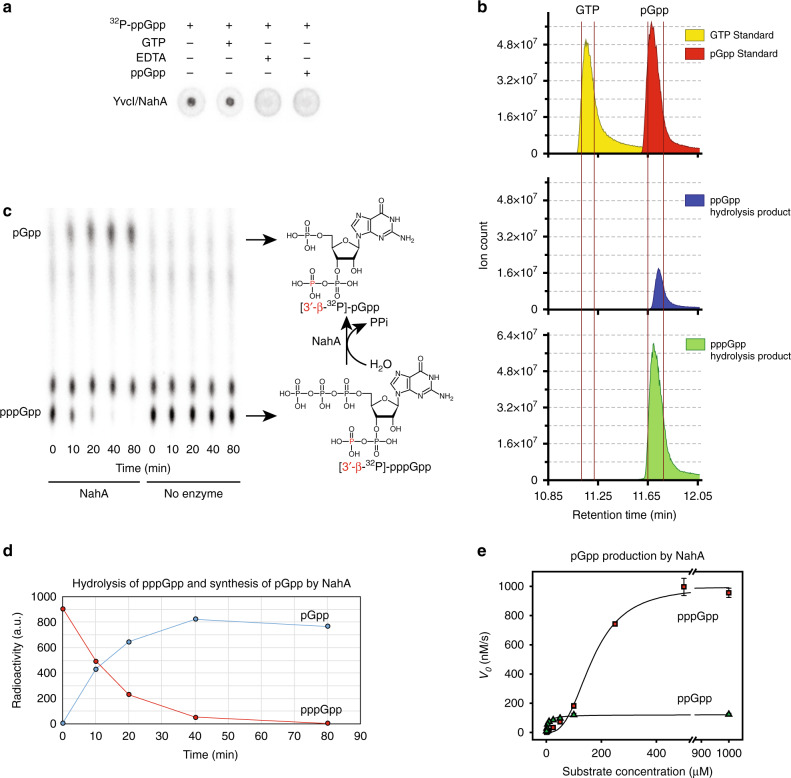
Fig. 2. NahA (YvcI) produces pGpp via (p)ppGpp hydrolysis.
a DRaCALA of [5′-α-32P]-ppGpp binding to B. subtilis HisMBP-tagged YvcI (NahA) overexpressed in E. coli cell lysate. Unlabeled GTP (100 μM), unlabeled ppGpp (100 μM) and EDTA (10 mM) were added as indicated. b Ion count vs. retention time curves from LC-MS of GTP and pGpp standards and NahA-catalyzed hydrolysis products from ppGpp and pppGpp. c TLC analysis of NahA activity over time with [3′-β-32P]-pppGpp. Expected NahA-catalyzed conversion of [3′-β-32P]-pppGpp to [3′-β-32P]-pGpp is shown on the right. Radiolabeled [3′-β-32P]-phosphorus atom is highlighted in red. d Quantitation of pppGpp and pGpp in the hydrolysis of pppGpp in c. e Initial velocity vs. pppGpp or ppGpp concentration curves for NahA synthesis of pGpp. Data were obtained by kinetic assay using radiolabels (see Methods section). Curves represent the best fit of the data from three independent experiments. Error bars represent standard error of the mean.