Abstract
Background
People who inject drugs (PWID) in rural areas of the United States have had limited access to syringe service programs (SSP). Rural SSP have recently surged, but accompanying research is lacking about PWID utilization, barriers, and preferences for SSP design and how those preferences vary by gender.
Methods:
Interviewer-administered surveys elicited information about utilization, barriers, and preferences for SSP design from 234 PWID recruited using respondent-driven sampling in Appalachian, Kentucky. Gender differences among reported barriers to utilizing SSP and preferences for program design were explored using Mantel-Haenszel chi-square tests.
Results:
Overall, 49% of PWID had ever utilized an SSP. The most common reasons for not utilizing an SSP were lack of awareness (23%), fear of being seen or disclosing drug use (19%), and lack of need (19%). The most preferred SSP design was located within a health department (74%) and operating during afternoon hours (66%). Men were more likely than women to prefer SSP in health departments (80% vs. 65%, p = 0.01), while more women than men preferred staffing by health department personnel (62% vs. 46%, p = 0.02). Women were less likely to favor evening hours (55% vs. 70%, p = 0.02). Fewer women wanted SSP nurses (78% vs. 90%, p = 0.01), social workers (11% vs. 24%, p = 0.01), or people who use drugs (20% vs 34%, p = 0.02) to staff SSP.
Conclusions:
Despite recent scale-up, SSP in Appalachia remain under-utilized. PWID were open to a range of options for SSP design and staffing, though there were variations by gender. Implementation research that identifies best strategies for tailored SSP scale-up in rural settings should be considered.
Keywords: Needle exchange programs, people who inject drugs, harm reduction, Appalachia, rural, gender
Background
New blood-borne infections, such as hepatitis c virus (HCV) and Human Immunodeficiency Virus (HIV), are on the rise in many rural regions in the United States due to injection drug use (IDU) (Klevens et al., 2012; Suryaprasad et al., 2014; Zibbell et al., 2015). Indeed, a 2017 analysis of US counties most vulnerable to HCV and HIV outbreaks due to IDU were overwhelmingly rural and concentrated in the Appalachian region of the US (Van Handel et al., 2016). Between 2006 and 2012, the number of acute HCV cases in central Appalachia grew dramatically by 364% among persons aged ≤30 years or older (Zibbell et al., 2015). HCV is often a precursor to impending increases in HIV infections, especially as IDU increases in rural areas (Vickerman et al., 2010). Reducing new HCV/HIV infections will require translating evidence-based approaches to alleviate the risks associated with sharing injection equipment. Evidence-based harm reduction programs, unfortunately, have been limited in settings that have become epicenters for IDU and related harms, particularly HCV/HIV. Within rural Appalachia, incidence of HCV infection has recently increased by nearly 400%, laying the foundation for HIV outbreaks—requiring a new frontier for harm reduction services (Lancaster et al., 2018; National Center for HIV/AIDS Viral Hepatitis STD and TB Prevention, 2015; Van Handel et al., 2016).
People who inject drugs (PWID) in rural areas of the United States have historically had limited access to syringe service programs (SSP) (Canary et al., 2017; Lancaster et al., 2018; Paquette & Pollini, 2018). As the rural opioid epidemic escalates, however, SSP are scaling up at an unprecedented pace within many rural settings, including rural Appalachia (Bixler et al., 2018). For example, within just five years in Kentucky, the state has gone from effectively prohibiting SSP to having 70 operational programs serving 60 of the state’s 120 counties (Kentucky Cabinet for Health and Family Services, 2019). SSP are an evidence-based and cost-effective approach to reducing parenteral exposure to HCV/HIV (Aspinall et al., 2014; Barbosa et al., 2019; Centers for Disease Control and Prevention, 2016; Platt et al., 2017; Wodak & Cooney, 2005). These programs provide sterile needles, syringes, and other injection equipment and pro-mote safer injection practices, as part of a package of harm reduction services (Centers for Disease Control and Prevention, 2016; Don C. Des Jarlais et al., 2009). Additional services can also include HCV/HIV testing and counseling, linkage to substance use disorder (SUD) treatment, and targeted education on reducing injection-related risks (Don C. Des Jarlais, 2017; Don C. Des Jarlais et al., 2009). Program designs can depend on community needs, client preferences, and operating budgets (Cooper et al., 2012; Davis et al., 2018; D. C. Des Jarlais et al., 2015). SSP are predominantly available through fixed locations such as health departments or mobile outreach, in which services are provided from cars or buses in various locations and times (D. C. Des Jarlais et al., 2015; Strike & Miskovic, 2018). The bulk of SSP is operating within large urban settings (D. C. Des Jarlais et al., 2015); though, the emergence of IDU-related harms in non-urban communities has resulted in the expansion of SSP to more remote settings (Canary et al., 2017).
Rural settings have traditionally lacked the health service infrastructure, that is more common in urban settings, and can lead to underserved specific subpopulations, such as women (Lancaster et al., 2018). Women may be especially vulnerable to experiencing barriers to healthcare utilization, specifically SSP, due to inaccessible service operation hours or locations, perceived or experienced stigma, childcare responsibilities, gender-related violence, and other gender inequalities that hinder access to SSP (El-Bassel & Strathdee, 2015; Iversen et al., 2015; Springer et al., 2015; Wagner et al., 2010). Gender relates to socially constructed characteristics, roles, and expectations that are informed by culture and society (World Health Organization, 2003). Addressing gender inequality as a barrier to healthcare utilization will require the development of gender-tailored SSP (Davey-Rothwell & Latkin, 2007; El-Bassel & Strathdee, 2015; Iversen et al., 2015; Springer et al., 2015; Wagner et al., 2010). SSP are generally tailored toward men as they primarily operate in public spaces and during the day, which does not provide an optimal space for safety and confidentiality for women who may fear partner violence or legal consequences (El-Bassel & Strathdee, 2015; Iversen et al., 2015; Pinkham & Malinowska-Sempruch, 2008). The gender difference in SSP preferences and use maybe even further amplified within rural settings, where research suggests gender gaps in utilization of other substance use related services (El-Bassel & Strathdee, 2015; Iversen et al., 2015; Meyer et al., 2019; Springer et al., 2015; Wagner et al., 2010). Understanding gender differences in preferences and use for SSP in rural areas will provide crucial insights to tailoring services for both men and women to improve utilization in these emerging opioid epicenters (Davis et al., 2018; Davis et al., 2019).
The legalization of SSP in 2015 within Kentucky has led to 60 counties opening SSP, collectively serving over 8,000 clients (Bixler et al., 2018). New SSP in rural Kentucky are generally operating within county health departments, opened from one to five days a week often during afternoon business hours, and staffed by nurses and health department personnel (Kentucky Cabinet for Health and Family Services, 2019). The SSP expansion is a critical effort to close the implementation chasm for harm reduction services in rural Kentucky. However, innovations to close this chasm can deepen health inequalities if the accessibility of their services are limited for certain subpopulations. Indeed, SSP designs that do not consider women’s preferences may continue to grow gender inequalities in drug-related healthcare service access for HCV/HIV. Here, we examined SSP utilization and barriers to utilization, as well as preferences for program design characteristics by gender to inform future SSP implementation in rural Appalachian, Kentucky.
Methods
Study design
Data were collected from people who use drugs (n = 278) during their baseline assessment in the ongoing, longitudinal Gateway2Health cohort, created as a part of the Kentucky Communities and Researchers Engaging to Halt the Opioid Epidemic (CARE2HOPE) study. The goal of CARE2HOPE is to build community-grounded, evidence-based responses to opioid use disorder and related harms in rural Kentucky. Eligibility criteria for the Gateway2Health cohort included being at least 18 years old, residing in one of the five Appalachian Kentucky counties, and having either used opioids to get high or injected any drug to get high in the prior 30 days.
Gateway2Health participants were recruited through respondent-driven sampling between February 2018 and April 2019 from five Appalachian counties in Eastern Kentucky. In order to avoid perpetuating stigma, we are not publishing the county names (Cloud et al., 2019; Fadanelli et al., 2019). These five counties were designated by the Appalachian Regional Commission as “Distressed Counties” based on economic indicators (e.g. unemployment, per capita income, and poverty rates) (Appalachian Regional Commission, 2020; Heckathorn, 1997, 2002). All five counties were classified as non-metropolitan and/or rural by the 2013 Urban Continuum Codes and 2010 Census (United States Department of Agriculture, 2013; US Census Bureau, 2010). The CDC has highlighted that the Appalachian region in Eastern Kentucky is considered highly vulnerable to experiencing or at-risk of outbreaks to HIV and HCV infections among PWID (Centers for Disease Control and Prevention, 2018). Additionally, two of the five counties for this study were ranked among the top 5% of counties in the US as being vulnerable to a surge in HIV and HCV infections among PWID (Van Handel et al., 2016). At the time of this study, SSP were operating out of health departments in three counties, from which approximately 80% of the sample was drawn. These SSP operate one day per week for fewer than four hours in the afternoon (Kentucky Cabinet for Health and Family Services, 2019).
Seeds for respondent-driven sampling were recruited from two sources: (1) a previous online survey study of young people who use drugs in the study area (described elsewhere) (Ballard et al., 2019) who consented to be contacted about future research, and (2) targeted outreach that involved distributing flyers at local businesses and organizations and hosting community cookouts that advertised the study. To qualify as Gateway2Health seeds, participants had to meet additional eligibility criteria and be “highly connected,” defined as reporting having used drugs with at least ten people in the past 30 days for women and at least 20 people in the past 30 days for men. These thresholds were determined based on a gender-stratified analysis of preliminary data from the online survey that found that network sizes of 10 and 20 demarcated the top quartile for women and men, respectively. Each of the seeds was given three numbered coupon cards and asked to provide them to peers who they thought may be interested in participating in the study. Seeds could distribute coupons to peers by giving them a paper copy or by sending a picture of the front and back of the card to them digitally (i.e. via text, email, or online messaging). For each coupon redeemed by an eligible recruit (up to three), the coupon distributor was offered $10 cash. Invited individuals who called or visited the study office, redeemed a valid coupon, and met inclusion criteria were invited to participate in the study. Upon completion of the interview, those recruited during the second wave were asked to refer up to three additional participants, and so on. Although participants were not encouraged to refer peers on the basis of SSP use, some homophily within the chains was observed on individuals’ past 30 day receipt of syringes from an SSP (homophily: 1.44).
All participants received copies of the consent form and provided signed documentation of informed consent. Participants completed interviewer-administered surveys and rapid HIV, HCV, and syphilis testing following the interview. Interviewers were extensively trained in nonjudgmental interviewing techniques to minimize the potential for social desirability bias. Participants received $25 for completing the 60-minute interviewer-administered survey and $20 for completing rapid HIV, HCV, and/or syphilis testing. The Institutional Review Board at the University of Kentucky approved the study protocol.
Measures
The primary dependent variable was SSP utilization. SSP utilization was determined using the survey question, “Have you ever personally visited a needle or syringe exchange program?” Those who answered yes were defined as having utilized an SSP and those who answered no were defined as never utilized an SSP. The primary independent variable was gender, in which participants were asked to self-report their gender, including male, female, or transgender. Based on the literature and sample size considerations, a parsimonious set of covariates were identified: age, education, number of times injecting in the last 30 days, and current drug of choice (Beletsky et al., 2014; Bluthenthal et al., 2007; Maurer et al., 2016; O’Keefe et al., 2018).
Participants who reported having never used an SSP were asked “Why haven’t you personally visited a needle or syringe exchange program?” and were able to select all potential barriers that applied. The potential barriers included: I can’t make it there during the times when it is open; I didn’t have any used needles that I could turn in to get new ones; I didn’t know there was one nearby; I was afraid that someone I know would see me there and found out that I was using drugs; I have never had a problem getting clean needles; I would rather just buy them from a pharmacy or from someone selling them; I did not want to be lectured to about my drug use and diseases; I was afraid that staff would judge me; I was afraid that law enforcement would be there or find out that I had gone; I did not want to be lectured to about my drug use and diseases; I was afraid that social services, like Department of Community Based Services (DCBS), would be there or find out that I had gone; I don’t understand how a syringe exchange works; and I couldn’t get transportation to get there. The list of potential barriers was developed based on previous literature as well as input from key informants, including SSP staff and local PWID (Beletsky et al., 2014; Grebely et al., 2017).
All participants were also asked their preferences on SSP design should a new local SSP be created. The series of questions focused on modality or location, hours, and staffing for SSP (see Table 3 for design features queried). The questions were developed in collaboration with key informants within the region as well as based on what was known about SSP designs employed in other domestic and international settings (Islam et al., 2008; O’Keefe et al., 2018). Participants were able to choose multiple response options based on their preferences.
Table 3.
Preferences for future syringe service programs by gender among people who inject drugs in Appalachian Kentucky.
| Total (n = 234) |
Men (n = 137) |
Women (n = 97) |
|||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | p-value | |
| Design | |||||||
| Health department | 172 | (74) | 109 | (80) | 63 | (65) | 0.01 |
| Health department in other counties | 127 | (54) | 82 | (60) | 45 | (46) | 0.04 |
| Mobile van | 147 | (63) | 86 | (61) | 61 | (63) | 0.99 |
| Vending machine | 142 | (61) | 80 | (58) | 62 | (64) | 0.40 |
| Delivery | 124 | (53) | 73 | (53) | 51 | (53) | 0.92 |
| Vending machine location* | |||||||
| Health department | 111 | (78) | 63 | (79) | 48 | (80) | 0.86 |
| Substance use treatment clinic | 93 | (65) | 53 | (66) | 40 | (67) | 0.96 |
| Gas station | 69 | (49) | 45 | (56) | 24 | (40) | 0.06 |
| Church | 29 | (20) | 14 | (18) | 15 | (25) | 0.28 |
| Hours | |||||||
| Morning, 8am-11am | 105 | (45) | 61 | (47) | 44 | (48) | 0.85 |
| Around noon, 11am −1pm | 113 | (48) | 70 | (53) | 43 | (47) | 0.33 |
| Afternoon, 1pm-5pm | 154 | (66) | 91 | (69) | 63 | (68) | 0.88 |
| Evening, after 5pm | 143 | (61) | 92 | (70) | 51 | (55) | 0.02 |
| Staffing | |||||||
| Nurses | 190 | (81) | 118 | (90) | 72 | (78) | 0.01 |
| Other health department staff | 157 | (67) | 100 | (76) | 57 | (62) | 0.02 |
| SUD counselors | 154 | (66) | 93 | (71) | 61 | (66) | 0.46 |
| Social workers | 42 | (18) | 32 | (24) | 10 | (11) | 0.01 |
| People who use drugs | 63 | (27) | 45 | (34) | 18 | (20) | 0.02 |
| People in recovery | 117 | (50) | 60 | (46) | 57 | (62) | 0.02 |
| Church members/leaders | 61 | (26) | 36 | (27) | 25 | (27) | 0.96 |
| People from out of town | 125 | (53) | 74 | (56) | 51 | (55) | 0.88 |
Question asked among participants who reported preferring vending machine SSP, n = 142.
SUD: Substance use disorder.
Analysis
Among the 278 people who use drugs, one person identified a transgender. Due to the limited number of people identifying as transgender, the analysis focused on those who identified as cisgender males or females referred to hereafter as “men” and “women” (n = 277). Of the 287, a total of 234 reported history of IDU in their lifetime. Therefore, analyses to assess the association of gender with the primary outcome, SSP utilization, were conducted on a sample size of 234. Poisson regression with robust variance estimates was used to estimate bivariable and multivariable prevalence ratios (PR) with 95% confidence intervals (CI), accounting for clustering within PWID descending from the same seed, while controlling for a parsimonious set of variables (Avery et al., 2019; Barros & Hirakata, 2003; Zou, 2004). Poisson regression with robust variance estimates is frequently used to estimate PRs in cross-sectional studies as odds ratios are known to overestimate risk when the prevalence of the outcome is greater than 10% (Barros & Hirakata, 2003; Zou, 2004). Multivariable models included the following variables: age, education, injected in the last 30 days, and current drug of choice to get high. Collinearity was evaluated in the model using Pearson’s correlation coefficient; none of the covariates showed a significant correlation (p-value ≥0.05).
Descriptive statistics summarize reported barriers to SSP utilization among those who reported not utilizing an SSP and preferences for SSP program design among all participants. Gender differences among preferences for SSP program design were explored using chi-square tests. When sample size allowed, county differences were also explored using chi-square tests to account for the variability of SSP access across our sample. A two-sided p-value of 0.05 was considered statistically significant for gender and county comparisons. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Participants were predominately white (97%) and 42% were women (Table 1). The median age was 35 years (IQR range: 29–41). Less than half (44%) had graduated from high school or completed a general education development (GED) certificate. Approximately 85% reported injecting drugs within the last 30 days. A quarter of our analytic sample reported injecting on average of 2 to 3 times a day. Nearly 20% reported injection more than 3 times a day. Less than half (41%) reported receiving syringes or needles from a SSP in the last 30 days and one-fifth (20%) receiving syringes or needles from someone else who utilized a SSP in the last 30 days. Approximately one-third (33%) reported methamphetamine, crystal meth, or amphetamine as their current drug of choice, followed by 29% reporting heroin as their preferred drug of choice to get high. The median age of the first injection was 25 years (IQR range: 19–30).
Table 1.
Characteristics of people who inject drugs in Appalachian Kentucky (N = 234).
| Total (n = 234) |
Men (n = 137) |
Women (n = 97) |
||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Age, years | ||||||
| 20–24 | 17 | (7.3) | 11 | (8.0) | 6 | (6.1) |
| 25–29 | 49 | (20.9) | 27 | (19.7) | 22 | (22.7) |
| 30–34 | 42 | (17.9) | 27 | (19.7) | 15 | (15.5) |
| 35–39 | 50 | (21.4) | 32 | (23.4) | 18 | (18.6) |
| ≤40 | 76 | (32.5) | 40 | (29.2) | 36 | (37.1) |
| Education* | ||||||
| Less than high school | 75 | (32.1) | 55 | (40.2) | 20 | (20.6) |
| High school diploma or GED | 102 | (43.6) | 58 | (42.3) | 44 | (45.4) |
| Some college | 40 | (17.1) | 17 | (12.4) | 23 | (23.7) |
| Associates degree, trade or technical school | 13 | (5.6) | 5 | (3.7) | 8 | (8.3) |
| Bachelors degree, other 4 year college degree or more | 3 | (1.3) | 1 | (0.7) | 2 | (2.0) |
| Race | ||||||
| White | 227 | (97.0) | 136 | (99.3) | 91 | (93.8) |
| African American or Black | 3 | (1.3) | 1 | (0.7) | 2 | (2.1) |
| Mixed race | 3 | (1.3) | 0 | (0.0) | 3 | (3.1) |
| Other | 1 | (0.4) | 0 | (0.0) | 1 | (1.0) |
| Injected drugs in last 30 days | ||||||
| Yes | 198 | (84.6) | 120 | (87.6) | 78 | (80.4) |
| No | 36 | (15.4) | 17 | (12.4) | 19 | (19.6) |
| Number of times on average injecting in last 30 days | ||||||
| More than 3 times a day | 43 | (18.4) | 29 | (21.2) | 14 | (14.4) |
| 2–3 times a day | 59 | (25.2) | 38 | (27.7) | 21 | (21.6) |
| Daily | 32 | (13.7) | 18 | (13.1) | 14 | (14.4) |
| More than weekly | 22 | (9.4) | 14 | (10.2) | 8 | (8.2) |
| Weekly | 14 | (6.0) | 4 | (2.9) | 10 | (10.3) |
| More than once in past 30 days | 15 | (6.4) | 7 | (5.1) | 8 | (8.2) |
| Once in past 30 days | 10 | (4.3) | 7 | (5.1) | 3 | (3.1) |
| Never or did not answer | 39 | (16.7) | 20 | (14.6) | 19 | (19.6) |
| Receipt of syringes or needles in last 30 days | ||||||
| Pharmacy | 2 | (0.9) | 2 | (1.5) | 0 | (0.0) |
| Syringe service program (SSP) | 95 | (40.6) | 62 | (45.3) | 33 | (34.0) |
| From someone else who received at SSP | 46 | (19.7) | 28 | (20.4) | 18 | (18.6) |
| Farm supply or veterinarian | 29 | (12.4) | 15 | (10.9) | 14 | (14.4) |
| Drug dealer or street syringe seller | 17 | (7.3) | 10 | (7.3) | 7 | (7.2) |
| Spouse, family member, sexual partner, or friend | 8 | (3.4) | 3 | (2.2) | 5 | (5.2) |
| Found them | 2 | (0.9) | 1 | (0.7) | 1 | (1.0) |
| Did not receive syringes or needles | 35 | (15.0) | 16 | (11.7) | 19 | (19.6) |
| Current drug of choice to get high | ||||||
| Heroin | 67 | (28.6) | 42 | (30.7) | 25 | (25.8) |
| Opiate painkillers | 42 | (17.9) | 19 | (13.8) | 23 | (23.7) |
| Methamphetamine, crystal meth, or amphetamine | 76 | (32.5) | 41 | (29.9) | 35 | (36.1) |
| Other*** | 49 | (20.9) | 35 | (25.6) | 15 | (14.4) |
| Age of first injection, years | ||||||
| ≤19 | 64 | (27.4) | 46 | (33.6) | 18 | (18.6) |
| 25–29 | 49 | (20.9) | 29 | (21.2) | 20 | (20.6) |
| 20–24 | 58 | (24.8) | 31 | (22.6) | 27 | (27.8) |
| ≤30 | 63 | (26.9) | 31 | (22.6) | 32 | (33.0) |
Missing data due to not knowing or refused to answer: Education, n 1.
Other includes: street fentanyl or carfentanil powder, synthetics like U47700, U4, or “Pink”, methadone, prescription anxiety drugs, cocaine or crack, gabapentin, and clonidine.
The proportion of those reporting ever using an SSP was 48% among women and 50% among men (Table 2). The majority (78%) of those reporting ever using an SSP also reported obtaining syringes or needles from a SSP in the past 30 days, however there were no meaningful differences by gender (results not shown). In adjusted multivariable analyses controlling for age, education, injected in the last 30 days, and current drug of choice, there was no statistically significant difference between men and women in their likelihood of having visited an SSP (PR: 1.04; 95% CI: 0.72, 1.51).
Table 2.
Prevalence and association of gender with syringe service programs utilization among people who inject drugs in Appalachian Kentucky.
| SSP utilization (N = 234) |
||||
|---|---|---|---|---|
| Utilized SSP (n = 115) | Never utilized SSP (n = 119) | Unadjusted PR (95% CI) | Adjusted PR (95% CI) | |
| Gender | ||||
| Men | 68 (50%) | 69 (50%) | 1.0 | 1.0 |
| Women | 47 (48%) | 50 (52%) | 0.98 (0.67, 1.42) | 1.04 (0.72, 1.51)a |
SSP syringe service program; PR prevalence ratio; CI confidence interval.
Adjusted for age, education, injected in last 30 days, and current drug of choice.
Among those who reported never using an SSP, the most common barriers reported were lack of awareness, stigma, and fear of breaches to confidentiality (Figure 1). The most frequently reported barrier was being unaware of the existence of a local SSP (23%), followed by never having a problem obtaining clean needles (19%), and fear of being seen or disclosing drug use (19%). The least commonly reported barrier was being unable to access the SSP during the current operating days and times (1%). There were no notable differences by gender.
Figure 1.
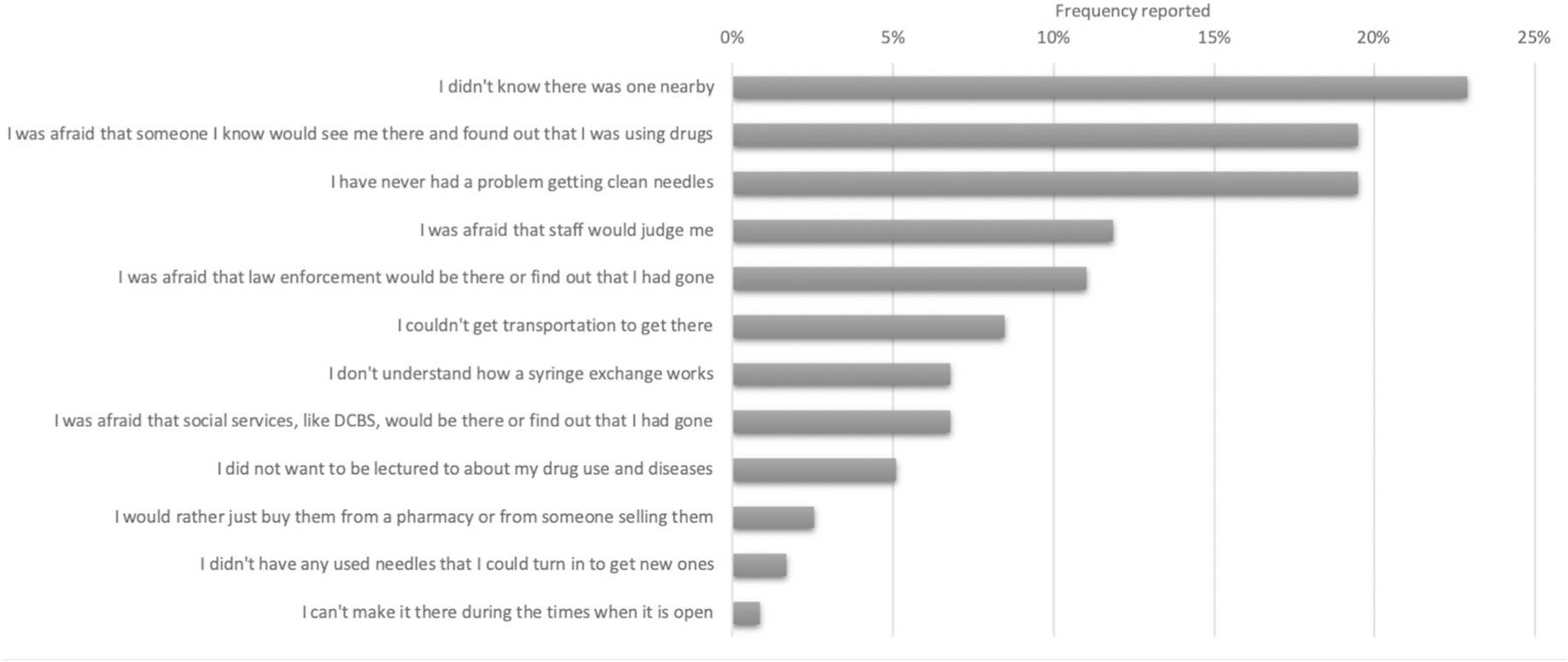
Reported barriers to utilizing syringe service program among people who inject drugs in Appalachian Kentucky.
DCBS = Department of Community Based Services.
Both men and women supported a range of SSP modalities and locations. The most popular SSP modality was one located within a local health department (74%), followed by mobile van (63%), vending machine (61%), health departments in other counties (54%), and home delivery (53%) (Table 3). PWID preferred that SSP operate during afternoon hours (66%) or evening hours (61%). Among those who preferred vending machines, the most preferred location was within health departments (78%), substance use disorder treatment clinics (65%), gas stations (49%), and the least preferred were churches (20%). Lower percentages of women than men wanted to locate SSP in their local health department (65% vs. 80%, p = 0.01) and health departments in other counties (46% vs. 60%, p = 0.04). Women were also less likely to favor evening hours (55% vs. 70%, p = 0.02). There were no statistically significant differences of SSP modalities and locations preferences by county (results not shown).
Gender differences were present for SSP staffing preferences. Overall across the sample, nurses were the most preferred for SSP staffing (81%) followed by other types of health department staff (67%) and SUD counselors (66%). Fewer women than men wanted nurses (78%% vs. 90%, p = 0.01), social workers (11% vs. 24%, p = 0.01), or people who use drugs (20% vs 34%, p = 0.02) to staff SSP. While significantly more women than men favored people in recovery (62% vs. 46%%, p = 0.02) to staff SSP. There were no statistical differences for SSP staffing preferences by county (results not shown).
Discussion
This study provides an enhanced understanding on the suboptimal utilization of SSP in this sample of high-risk PWID and identifies gender differences in modifiable SSP design preferences in a rural area with an escalating opioid epidemic. Several benefits of SSP exists for reducing HCV/HIV risk as they are a place to receive clean needles and additional services, such as testing, yet an unmet need exists among PWID in our sample with less than half receiving the direct benefits of SSP utilization. Additionally, there was no statistically significant difference in the proportion of men and women who had used the local SSP, but for both groups, utilization was inadequate. Among those who had not used the SSP, lack of awareness, stigma, and fear of breaches to confidentiality were the primary barriers.
Both men and women supported a range of SSP design characteristics. Significantly more women than men in our study preferred health-department based SSP staffed by health-department personnel. In this region, however, all SSP are operated from health departments and staffed by health department personnel. Although not included in this study, preferences for gender of SSP staff should also be explored as a potential SSP design characteristic. Preferences for modality and staffing were more heterogeneous among women than men, indicating that offering a variety of designs may be necessary to meet women’s needs. These gender differences emphasize possible improvements to tailor evidence-based interventions and practices, such as SSP, to potentially improve access for people to engage with their preferred programs (Mittman, 2012; Powell et al., 2017).
Women experience a unique set of social and structural factors that influence their healthcare service utilization that can preclude SSP utilization (El-Bassel & Strathdee, 2015; Springer et al., 2015; Wagner et al., 2010). In Kentucky, health departments offer a range of wellness initiatives for pregnant women, mothers, and children. Although additional qualitative research is needed to fully explore reasons for women’s decreased preference of SSP located in health departments, fear of being seen at the SSP by the same people who assist with maternal and child healthcare could play a role. Within studies in similar settings, women have reported their fear that staff will file a report with community-based services in charge of child welfare (El-Bassel & Strathdee, 2015; Iversen et al., 2015; Pinkham & Malinowska-Sempruch, 2008). Further implementation evaluations are needed to understand better women’s concerns related to SSP use and to identify novel approaches in tailoring SSP for women.
The establishment of SSP across rural Kentucky has been unprecedented in its pace (Bixler et al., 2018). The harm reduction movement in Kentucky gained momentum after a rural community in neighboring Indiana experienced a historic HIV outbreak in 2014 and after a subsequent analysis revealed that many of Kentucky’s counties were highly vulnerable to a similar outbreak of HIV and HCV among PWID (Conrad et al., 2015; Van Handel et al., 2016). Despite advances in harm reduction in Kentucky, data from PWID in this study suggest that these services have not yet closed the implementation chasm and remain under-utilized by PWID due primarily to lack of awareness and stigma; these factors are not unrelated. Negative attitudes toward SSP and stigma against SUD among community members at large can discourage health officials from adequately advertising SSP for fear that community pushback could jeopardize the program’s existence. In Kentucky, the county health board, county fiscal court, and city council for the jurisdiction where the SSP operates must approve the establishment of an SSP before it can operate; the latter two entities are comprised of elected officials who are influenced by public opinion and who could potentially push to revoke approval for the program. For example, in nearby West Virginia, the largest SSP opened in 2015, however, was later shut down in 2018 likely as the result of public criticism from the local mayor (Appalachia Health News, 2018). Addressing community stigma around harm reduction and decreasing vulnerability of programs’ existence to changes in public opinion will be critical before SSPs’ full public health potential can be realized. In the interim, strategies to advertise SSP to those who need it most without increasing public awareness might be required.
Reported barriers to SSP utilization within our sample differ from previous examinations among PWID in nearby Appalachia West Virginia and North Carolina, which signals the importance of tailoring SSP within multiple Appalachian settings. SSP barriers among PWID who had not previously utilized SSP in Kentucky were consistent between women and men and included being unaware of SSP availability and fear of social stigma. Among a sample of PWID utilizing SSP in West Virginia, fear of police or potential arrest was the most frequently reported barrier to obtain clean needles, either through an SSP or pharmacy (Davis et al., 2019). Similarly, in North Carolina, fear of police and criminalization impede the effectiveness of SSP in both urban and rural areas (Cloud et al., 2018). These differences may suggest differences in interactions with local law enforcement, but without further qualitative research, this remains unknown. West Virginia, in comparison to Kentucky, has significantly fewer SSP across the state, and PWID using SSP in Appalachia West Virginia have noted the proximity of an SSP site and police department (Davis et al., 2018). In the counties included in this study, the local police and sheriff departments are approximately a half-mile or less from the SSP but not clearly visible from the SSP entrance. More research is needed to determine if fear of police plays a prominent role in SSP utilization in Kentucky. The reports of barriers from our sample are limited to only those who had not previously utilized SSP in their lifetime. Given this, we missed the opportunity to examine more recent barriers among the small subset who previously utilized SSP but have not in the prior month. Further evaluations that specifically examine additional current SSP barriers within multiple Appalachian settings are warranted to directly inform SSP designs.
Our findings may have implications for future implementation science research to integrate SSP and SUD treatment services. Women in our sample were significantly more likely than men to prefer people in recovery as SSP staff, illuminating a potential useful entry point for engaging women in discussions about recovery and SUD treatment. Approximately twice as many women as men are referred to SUD treatment through community agencies, such as welfare, mental health, and other health care providers (Greenfield et al., 2010). While a primary purpose of SSP is to prevent HCV/HIV, SSP are often an opportunity for SUD treatment referrals (Heimer, 1998). Although SSP participants have expressed interest and readiness for treatment, entry is generally low when referrals are provided (Henderson et al., 2003; Kidorf et al., 2005). Staffing SSP with peers in recovery who can not only offer referrals but also recovery coaching, mentoring, and linkage to other support organizations, including local housing, local treatment providers, and other social support services could hold promise for enhancing women’s linkage and entry to substance use treatment (Ashford et al., 2018). Further research within Appalachia Kentucky is needed to explore interest and readiness for treatment among those accessing SSP.
We directly asked participants on SSP designs, which allowed for deconstructed preferences for each design characteristics. However, more rigorous preference assessments informed by stated-preference theory should be conducted to gain an enhanced understanding of preferences and decision making within the context of various characteristics (Bridges et al., 2011; Street & Burgess, 2007). Data were also collected from interviewer-administered surveys, and therefore, interviewers were trained to minimize the potential for recall and social desirability bias. Furthermore, our sample size limited our ability to examine the heterogeneity of factors influencing preferences beyond gender differences. Lastly, SSP barriers were assessed only among those who reported not utilizing SSP in their lifetime. Future assessments, including qualitative research to further explore barriers regardless of lifetime SSP utilization, could provide greater insights on potential diversity in barriers among PWID in rural settings
Conclusion
The benefits of SSP are well-established, yet these programs are underutilized among PWID in our sample despite the recent SSP scale-up in the region. To increase reach, interventions are needed to reduce stigma and improve awareness of SSP among rural PWID. Further, these findings indicate that rural PWID are interested in a variety of SSP designs beyond the current fixed-site models, including mobile programs, vending machine models, and home delivery. Agencies establishing rural SSP should consider innovative designs that account for the heterogeneity in needs and preferences among rural PWID. The latter may be especially important to avoid a potential gender gap in utilization as women were significantly less likely than men to prefer the current health department based design. Implementation research that seeks to understand the best strategies to tailor SSP scale-up by gender preferences in rural settings should be conducted.
Acknowledgements
We would like to acknowledge the participants involved in this study for sharing their information and experiences with us, as well as the community-based staff who collected the data and community-academic partnership coalitions who provided helped to guide study and survey design.
Funding
This work was supported by National Institute on Drug Abuse (K01DA048174: PI Lancaster; UG3DA044798: PIs Young and Cooper; and UG3DA044798 02S1: PIs Young and Cooper).
Abbreviations:
- HCV
Hepatitis C virus
- HIV
Human Immunodeficiency Virus
- IDU
Injection drug use
- PWID
People who inject drugs
- SUD
Substance use disorder
- SSP
Syringe service programs
Footnotes
Declaration of interest
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the article.
Availability of data and materials
The data analyzed in this current study is not publicly available due to it containing information that could compromise research participant privacy and consent but are available from the corresponding author on reasonable request.
Consent for publication
All authors have read the manuscript and have consented this submission for publication.
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board at the University of Kentucky. All participants provided informed, written consent prior to participation.
References
- Appalachia Health News. (2018). Diving Deep into Harm Reduction Part 1: Why W.Va.’s Largest Needle Exchange Closed. Retrieved from https://www.wvpublic.org/post/diving-deep-harm-reduction-part-1-why-wva-s-largest-needle-exchange-closed#stream/0
- Appalachian Regional Commission. (2020). ARC-Designated Distressed Counties, Fiscal Year Retrieved from https://www.arc.gov/program_areas/ARCDesignatedDistressedCountiesFiscalYear2020.asp
- Ashford RD, Curtis B, & Brown AM (2018). Peer-delivered harm reduction and recovery support services: Initial evaluation from a hybrid recovery community drop-in center and syringe exchange program. Harm Reduction Journal, 15(1), 52–52. 10.1186/s12954-018-0258-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, Palmateer N, Doyle JS, Hellard ME, & Hutchinson SJ (2014). Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: A systematic review and meta-analysis. International Journal of Epidemiology, 43(1), 235–248. 10.1093/ije/dyt243 [DOI] [PubMed] [Google Scholar]
- Avery L, Rotondi N, McKnight C, Firestone M, Smylie J, & Rotondi M (2019). Unweighted regression models perform better than weighted regression techniques for respondent-driven sampling data: Results from a simulation study. BMC Medical Research Methodology, 19(1), 202 10.1186/s12874-019-0842-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard AM, Cooper HL, & Young AM (2019). Web-based eligibility quizzes to verify opioid use and county residence among rural young adults: Eligibility screening results from a feasibility study. JMIR Research Protocols, 8(6), e12984 10.2196/12984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C, Fraser H, Hoerger TJ, Leib A, Havens JR, Young A, Kral A, Page K, Evans J, Zibbell J, Hariri S, Vellozzi C, Nerlander L, Ward JW, & Vickerman P (2019). Cost-effectiveness of scaling-up HCV prevention and treatment in the United States for people who inject drugs. Addiction (Abingdon, England), 114(12), 2267–2278. 10.1111/add.14731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros AJ, & Hirakata VN (2003). Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Medical Research Methodology, 3, 21 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletsky L, Heller D, Jenness SM, Neaigus A, Gelpi-Acosta C, & Hagan H (2014). Syringe access, syringe sharing, and police encounters among people who inject drugs in New York City: A community-level perspective. The International Journal on Drug Policy, 25(1), 105–111. 10.1016/j.drugpo.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler D, Corby-Lee G, Proescholdbell S, Ramirez T, Kilkenny ME, LaRocco M, Childs R, Brumage MR, Settle AD, Teshale EH, & Asher A (2018). Access to syringe services prorams - Kentucky, North Carolina, and West Virginia, 2013–2017. MMWR. Morbidity and Mortality Weekly Report, 67(18), 529–532. 10.15585/mmwr.mm6718a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthenthal RN, Anderson R, Flynn NM, & Kral AH (2007). Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug and Alcohol Dependence, 89(2–3), 214–222. 10.1016/j.drugalcdep.2006.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, & Mauskopf J (2011). Conjoint analysis applications in health-a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research, 14(4), 403–413. 10.1016/j.jval.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Canary L, Hariri S, Campbell C, Young R, Whitcomb J, Kaufman H, & Vellozzi C (2017). Geographic disparities in access to syringe services programs among young persons with Hepatitis C Virus infection in the United States. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 65(3), 514–517. 10.1093/cid/cix333 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2016). HIV and Injection Drug Use: Syringe Services Programs for HIV Prevention. Retrieved from https://www.cdc.gov/vitalsigns/pdf/2016-12-vitalsigns.pdf
- Centers for Disease Control and Prevention. (2018). Vulnerable Counties and Jurisdictions Experiencing or At-Risk of Outbreaks. Retrieved from https://www.cdc.gov/pwid/vulnerable-counties-data.html
- Cloud DH, Castillo T, Brinkley-Rubinstein L, Dubey M, & Childs R (2018). Syringe decriminalization advocacy in red states: Lessons from the North Carolina harm reduction coalition. Current HIV/AIDS Reports, 15(3), 276–282. 10.1007/s11904-018-0397-9 [DOI] [PubMed] [Google Scholar]
- Cloud DH, Ibragimov U, Prood N, Young AM, & Cooper HLF (2019). Rural risk environments for Hepatitis C among young adults in Appalachian Kentucky. The International Journal on Drug Policy, 72, 47–54. 10.1016/j.drugpo.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C, Bradley HM, Broz D, Buddha S, Chapman EL, Galang RR, Hillman D, Hon J, Hoover KW, Patel MR, Perez A, Peters PJ, Pontones P, Roseberry JC, Sandoval M, Shields J, Walthall J, Waterhouse D, Weidle PJ, Wu H, & Duwve JM, Centers for Disease Control and Prevention (CDC). (2015). Community outbreak of HIV infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR. Morbidity and Mortality Weekly Report, 64(16), 443–444. [PMC free article] [PubMed] [Google Scholar]
- Cooper H, Des Jarlais D, Ross Z, Tempalski B, Bossak BH, & Friedman SR (2012). Spatial access to sterile syringes and the odds of injecting with an unsterile syringe among injectors: A longitudinal multilevel study. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 89(4), 678–696. 10.1007/s11524-012-9673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey-Rothwell MA, & Latkin CA (2007). Gender differences in social network influence among injection drug users: Perceived norms and needle sharing. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 84(5), 691–703. 10.1007/s11524-007-9215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Davidov D, Kristjansson AL, Zullig K, Baus A, & Fisher M (2018). Qualitative case study of needle exchange programs in the Central Appalachian region of the United States. PLoS One, 13(10), e0205466 10.1371/journal.pone.0205466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Kristjansson AL, Davidov D, Zullig K, Baus A, & Fisher M (2019). Barriers to using new needles encountered by rural Appalachian people who inject drugs: Implications for needle exchange. Harm Reduction Journal, 16(1), 23 10.1186/s12954-019-0295-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC (2017). Harm reduction in the USA: The research perspective and an archive to David Purchase. Harm Reduction Journal, 14(1), 51–51. 10.1186/s12954-017-0178-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, McKnight C, Goldblatt C, & Purchase D (2009). Doing harm reduction better: Syringe exchange in the United States. Addiction (Abingdon, England), 104(9), 1441–1446. 10.1111/j.1360-0443.2008.02465.x [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, & Holtzman D (2015). Syringe service programs for persons who inject drugs in urban, suburban, and rural areas - United States, 2013. MMWR. Morbidity and Mortality Weekly Report, 64(48), 1337–1341. 10.15585/mmwr.mm6448a3 [DOI] [PubMed] [Google Scholar]
- El-Bassel N, & Strathdee SA (2015). Women who use or inject drugs: An action agenda for women-specific, multilevel, and combination HIV prevention and research. Journal of Acquired Immune Deficiency Syndromes (1999), 69 Suppl 2(2), S182–S190. 10.1097/QAI.0000000000000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadanelli M, Cloud DH, Ibragimov U, Ballard AM, Prood N, Young AM, & Cooper HLF (2019). People, places, and stigma: A qualitative study exploring the overdose risk environment in rural Kentucky. International Journal on Drug Policy, 102588 10.1016/j.drugpo.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Dore GJ, Morin S, Rockstroh JK, & Klein MB (2017). Elimination of HCV as a public health concern among people who inject drugs by 2030 - What will it take to get there? Journal of the International AIDS Society, 20(1), 22146 10.7448/IAS.20.1.22146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, & Brady KT (2010). Substance abuse in women. The Psychiatric Clinics of North America, 33(2), 339–355. 10.1016/j.psc.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn DD (1997). Respondent-driven sampling: A new approach to the study of hidden populations. Social Problems, 44(2), 174–199. 10.2307/3096941 [DOI] [Google Scholar]
- Heckathorn DD (2002). Respondent-driven sampling II: Deriving valid population estimates from chain-referral samples of hidden populations. Social Problems, 49(1), 11–34. 10.1525/sp.2002.49.1.11 [DOI] [Google Scholar]
- Heimer R (1998). Can syringe exchange serve as a conduit to substance abuse treatment? Journal of Substance Abuse Treatment, 15(3), 183–191. 10.1016/S0740-5472(97)00220-1 [DOI] [PubMed] [Google Scholar]
- Henderson LA, Vlahov D, Celentano DD, & Strathdee SA (2003). Readiness for cessation of drug use among recent attenders and nonattenders of a needle exchange program. Journal of Acquired Immune Deficiency Syndromes (1999), 32(2), 229–237. 10.1097/00126334-200302010-00017 [DOI] [PubMed] [Google Scholar]
- Islam M, Wodak A, & Conigrave KM (2008). The effectiveness and safety of syringe vending machines as a component of needle syringe programmes in community settings. The International Journal on Drug Policy, 19(6), 436–441. 10.1016/j.drugpo.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Iversen J, Page K, Madden A, & Maher L (2015). HIV, HCV and health-related harms among women who inject drugs: Implications for prevention and treatment. JAIDS: Journal of Acquired Immune Deficiency Syndromes, 69(0 1), S176–S181. 10.1097/QAI.0000000000000659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentucky Cabinet for Health and Family Services. (2019). Syringe Exchange Programs. Retrieved from https://chfs.ky.gov/agencies/dph/dehp/hab/Pages/kyseps.aspx
- Kidorf M, Disney E, King V, Kolodner K, Beilenson P, & Brooner RK (2005). Challenges in motivating treatment enrollment in community syringe exchange participants. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 82(3), 456–467. 10.1093/jurban/jti091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Hu DJ, Jiles R, & Holmberg SD (2012). Evolving epidemiology of Hepatitis C virus in the United States. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 55(Suppl 1), S3–S9. 10.1093/cid/cis393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster KE, Malvestutto CD, Miller WC, & Go VF (2018). Commentary on Fraser et al. (2018): Evidence base for harm reduction services-the urban-rural divide. Addiction (Abingdon, England), 113(1), 183–184. 10.1111/add.14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer LA, Bass SB, Ye D, Benitez J, Mazzella S, & Krafty R (2016). Trend analyses of users of a syringe exchange program in Philadelphia, Pennsylvania: 1999–2014. AIDS and Behavior, 20(12), 2922–2932. 10.1007/s10461-016-1393-y [DOI] [PubMed] [Google Scholar]
- Meyer JP, Isaacs K, El-Shahawy O, Burlew AK, & Wechsberg W. (2019). Research on women with substance use disorders: Reviewing progress and developing a research and implementation roadmap. Drug and Alcohol Dependence, 197, 158–163. 10.1016/j.drugalcdep.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittman BS (2012). 19 Implementation Science in Health Care. Dissemination and implementation research in health: translating science to practice, 1400. [Google Scholar]
- National Center for HIV/AIDS Viral Hepatitis STD and TB Prevention. (2015). Ohio-2015 State Health Profile. Retrieved from https://www.cdc.gov/nchhstp/stateprofiles/pdf/ohio_profile.pdf
- O’Keefe D, Aitken C, Scott N, & Dietze P (2018). Analysis of time of drug use according to needle and syringe program operating hours in Melbourne, Australia: Effects on individual-level needle and syringe coverage. Drug and Alcohol Dependence, 191, 259–265. 10.1016/j.drugalcdep.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Paquette CE, & Pollini RA (2018). Injection drug use, HIV/HCV, and related services in nonurban areas of the United States: A systematic review. Drug and Alcohol Dependence, 188, 239–250. 10.1016/j.drugalcdep.2018.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham S, & Malinowska-Sempruch K (2008). Women, harm reduction and HIV. Reproductive Health Matters, 16(31), 168–181. 10.1016/S0968-8080(08)31345-7 [DOI] [PubMed] [Google Scholar]
- Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, Jordan A, Degenhardt L, Hope V, Hutchinson S, Maher L, Palmateer N, Taylor A, Bruneau J, & Hickman M (2017). Needle syringe programmes and opioid substitution therapy for preventing Hepatitis C transmission in people who inject drugs. The Cochrane Database of Systematic Reviews, 9, CD012021. 10.1002/14651858.CD012021.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell BJ, Beidas RS, Lewis CC, Aarons GA, McMillen JC, Proctor EK, & Mandell DS (2017). Methods to improve the selection and tailoring of implementation strategies. The Journal of Behavioral Health Services & Research, 44(2), 177–194. 10.1007/s11414-015-9475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Larney S, Alam-Mehrjerdi Z, Altice FL, Metzger D, & Shoptaw S (2015). Drug treatment as HIV prevention among women and girls who inject drugs from a global perspective: Progress, gaps, and future directions. JAIDS: Journal of Acquired Immune Deficiency Syndromes, 69(0 1), S155–S161. 10.1097/QAI.0000000000000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street DJ, & Burgess L (2007). The construction of optimal stated choice experiments: Theory and methods (Vol. 647). John Wiley & Sons. [Google Scholar]
- Strike C, & Miskovic M (2018). Scoping out the literature on mobile needle and syringe programs-review of service delivery and client characteristics, operation, utilization, referrals, and impact. Harm Reduction Journal, 15(1), 6 10.1186/s12954-018-0212-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryaprasad AG, White JZ, Xu F, Eichler B-A, Hamilton J, Patel A, Hamdounia SB, Church DR, Barton K, Fisher C, Macomber K, Stanley M, Guilfoyle SM, Sweet K, Liu S, Iqbal K, Tohme R, Sharapov U, Kupronis BA, Ward JW, & Holmberg SD (2014). Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clinical infectious Diseases: An official publication of the Infectious Diseases Society of America, 59(10), 1411–1419. 10.1093/cid/ciu643 [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture. (2013). Rural-Urban Continuum Codes. Retrieved from https://www.ers.usda.gov/dataproducts/rural-urban-continuum-codes.aspx.
- US Census Bureau. (2010). 2010 Census. US Census Bureau. [Google Scholar]
- Van Handel MM, Rose CE, Hallisey EJ, Kolling JL, Zibbell JE, Lewis B, Bohm MK, Jones CM, Flanagan BE, Siddiqi A-E-A, Iqbal K, Dent AL, Mermin JH, McCray E, Ward JW, & Brooks JT (2016). County-level vulnerability assessment for rapid dissemination of HIV or HCV infections among persons who inject drugs, United States. Journal of Acquired Immune Deficiency Syndromes (1999), 73(3), 323–331. 10.1097/QAI.0000000000001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman P, Hickman M, May M, Kretzschmar M, & Wiessing L (2010). Can Hepatitis C virus prevalence be used as a measure of injection-related human immunodeficiency virus risk in populations of injecting drug users? An ecological analysis. Addiction, 105(2), 311–318. 10.1111/j.1360-0443.2009.02759.x [DOI] [PubMed] [Google Scholar]
- Wagner KD, Lankenau SE, Palinkas LA, Richardson JL, Chou CP, & Unger JB (2010). The perceived consequences of safer injection: An exploration of qualitative findings and gender differences. Psychology, Health & Medicine, 15(5), 560–573. 10.1080/13548506.2010.498890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodak A, & Cooney A (2005). Effectiveness of sterile needle and syringe programmes. International Journal of Drug Policy, 16, 31–44. 10.1016/j.drugpo.2005.02.004 [DOI] [Google Scholar]
- World Health Organization. (2003). Integrating gender into HIV/AIDS programmes. Retrieved from http://www.who.int/hiv/pub/prev_care/en/IntegratingGender.pdf
- Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, … Holtzman D (2015). Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morbidity and Mortality Weekly Report, 64(17), 453–458. [PMC free article] [PubMed] [Google Scholar]
- Zou G (2004). A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology, 159(7), 702–706. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]


