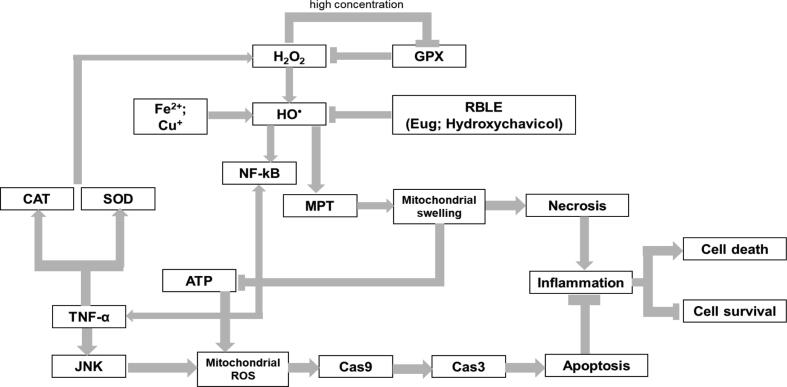
Fig. 5.
Proposed mechanism on how RBLE could act as a hepatoprotective agent in liver injury model·H2O2 is transformed into hydroxyl radical (HO*) through Fenton reaction with the presence of transition metal such as iron and copper. Under normal condition, H2O2 is neutralized by Glutathione Peroxidase (GPX) enzymes activity through oxidation of GSH into GSSG. It seemed that excessive H2O2 lead to down regulation of GPX gene expression. Hydroxyl radical activates NFkB that lead to TNF-α production. TNF/TNFR engagement induces production of catalase (CAT) and superoxide dismutase (SOD) and thus increasing extracellular H2O2. Meanwhile, TNF-α also induced JNK signaling pathways, resulting in increased mitochondrial ROS and lead to activation of Caspase 9 and apoptosis executioner, Caspase3. On the other hand, high amount of HO* triggers mitochondrial permeability transition (MPT), causing mitochondria to swell, and suppress ATP production. This pathway lead to unregulated cell death, necrosis; increase inflammation; and promote cell death. Lack of ATP also affects apoptosis pathway and switch it into necrosis instead. If this condition left untreated, liver injury could take place. Treatment with RBLE could neutralized HO* in cells and thus lowering necrosis, shifted the cells death pathway to apoptosis which induce lower inflammation response instead. Subsequently, RBLE treatment could increase the survival of hepatic cells.