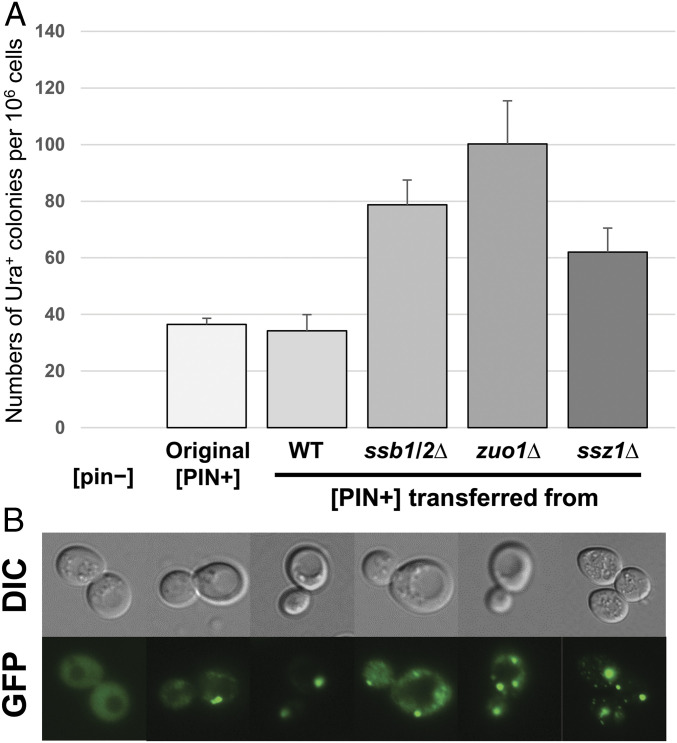
Fig. 5.
Alteration of [PIN+] prion by ssb1/2Δ, zuo1Δ, and ssz1Δ. (A) The efficiency of [PSI+] induction was slightly elevated in a WT strain carrying [PIN+] from ssb1/2Δ, zuo1Δ, or ssz1Δ. Into each mutant strain (MS515, MS527, MS510) and the WT (MS327), all made [pin−], the [PIN+] from from BY4742 (=“Original [PIN+]”) was introduced by cytoduction. The mutants showed high [PSI+] generation (Table 1). Then the [PIN+] in each mutant and WT strain was transferred back into MS173 (previously made [pin-] by guanidine curing) by cytoduction. The “[pin−]” strain is MS173 cured of [PIN+]. In these strains, all with WT genome but [PIN+] from different sources, [PSI+] was induced by overproduction of Sup35NM in 2% (wt/vol) raffinose, 2% (wt/vol) galactose minimal medium. Cells were plated on −Ura plates, and arising Ura+ ([PSI+]) clones were tested for GuHCl curability using transient growth on plates containing 5 mM guanidine. (B) The formation of Rnq1-GFP aggregates in different [PIN+]-carrying strains. BY4742 [pin−], BY4742 [PIN+], MS562, MS563, MS564, and MS565 were transformed with pM60 (pCEN LEU2) expressing ADH1 promoted Rnq1-GFP. Transformants were directly used for fluorescence confocal microscopy observation (magnification 1500×).