Table 1.
The molecular details of five shortlisted MB compounds and the reference compounds, produced by molecular docking.
| S. No. | Ligand | 3D structures | SMILES Format | Binding Score | H-bond residues |
|---|---|---|---|---|---|
| 1 | MB_183 |  |
C1(NC[NH]C1C(=O)NC1[NH]C2CCCCC2N1)C(=O)NC1CCC(C)CC1C | −7.4 kcal/mol | Asn142 |
| 2 | MB_241 | 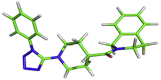 |
[C@H]1(CCN(CC1)[C@@H]1NNNN1C1CCCCC1)C(=O)N1CCC2CCCCC2C1 | −7.6 kcal/mol | Leu141, Ser144 and Glu166 |
| 3 | MB_250 |  |
C1(CCCC(C1NC(=O)C1CC(ON1)C1CCCCC1)O)C1OC2CCCC(C2N1)C(O)O | −8.8 kcal/mol | His41, Leu141 and His163 |
| 4 | MB_266 | 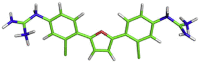 |
C1(OC(CC1)C1CCC(CC1CL)[NH2]C(=[NH2])[NH3])C1CCC(CC1CL)[NH2]C(=[NH2])[NH3] | −8.9 kcal/mol | Thr24, Thr25, Thr45, Ser46, Asn142 and Phe140 |
| 5 | MB_380 | 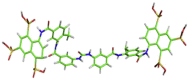 |
N(C(=O)NC1CCCC(C1)C(=O)NC1CC(CCC1C)C(=O)NC1CCC(C2CC(CC(C12)S(=O)(=O)O)S(=O)(=O)O)S(=O)(=O)O)C1CC(CCC1)C(=O)NC1C(C)CCC(C1)C(=O)NC1C2C(CC(CC2C(CC1)S(=O)(=O)O)S(=O)(=O)O)S(=O)(=O)O | −9.2 kcal/mol | Thr24, Thr25, Ser46, Asn142, and Gln189 |
| 6 | N3 inhibitor | 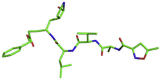 |
CC1=CC(=NO1)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](\C C/C(=O)OCC1=CC CC C1)C[C@@H]1C(NCC1) = O | −8.3 kcal/mol | Asn142, Gly143, Glu166, Leu167, and Gln189 |
| 7 | Boceprevir | 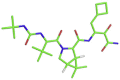 |
[H][C@]12CN([C@H](C(=O)NC(CC3CCC3)C(=O)C(N) O)[C@@]1([H])C2(C)C)C(=O)[C@@H](NC(=O)NC(C)(C)C)C(C)(C)C | −6.8 kcal/mol | His41, Tyr54, Met49, Gly143, and His164 |
