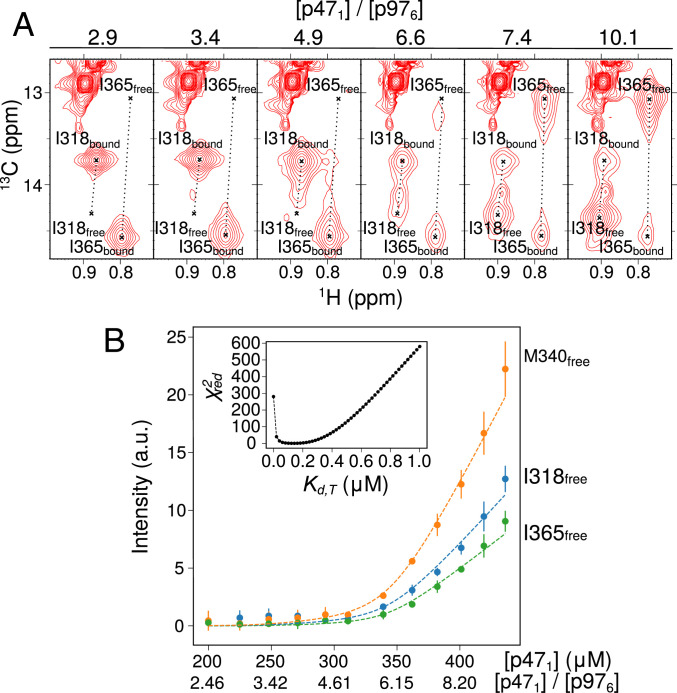
Fig. 6.
p47 binds to p97ND1Lapo to form a 6:6 complex. (A) Selective regions of 13C-1H HMQC spectra of [U-2H,IM-13CH3]p47 as a function of [p471]/[U-2H-p97ND1Lapo], highlighting UBX domain residues I318 and I365 of p47, with crosshairs indicating the peak positions in the free and bound states. (B) Intensities of cross-peaks derived from methyl groups of I365 (green), I318 (blue), and M340 (orange) in the p47 free state, upon addition of [U-2H,IM-13CH3]p47 into a preformed solution of [U-2H, IM-13CH3]p47 and [U-2H]p97ND1Lapo ([p47]:[p97]∼2.5:6). Dashed lines represent best fits to a model that takes into account both bipartite (UBX+SHPC) and tripartite (UBX+SHPC+SHPN) p47 binding, as described in SI Appendix, Materials and Methods. The titration data were fit with Kd,B = 0.55 μM (obtained from ITC for the bipartite binding of p47171–370 to p97ND1Lapo) to yield Kd,T = 0.15 ± 0.02 μM. Inset shows reduced χ2 values as a function of Kd,T. Data were recorded at 23.5 T (40 °C). a.u., arbitrary units.