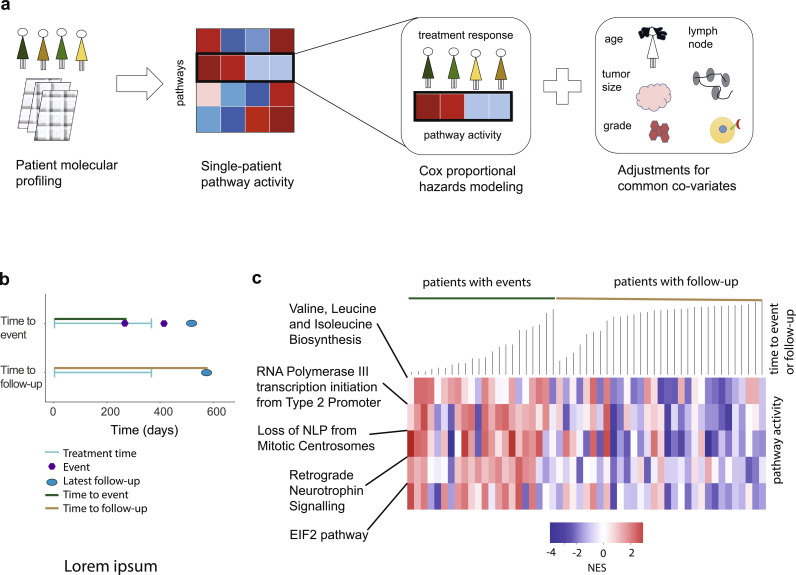
Fig. 2.
Training phase: pathway-centric approach identifies five biological pathways that govern tamoxifen response. (a) Schematic representation of the Testing phase of our approach: (left) patient molecular profiles are collected and analyzed; (middle) pathway activities are estimated in each patient using single-patient pathway enrichment analysis; (right) pathway activities are associated with response to tamoxifen using Cox proportional hazards modeling and are adjusted to common covariates, including age, tumor grade, tumor size (> 2 cm vs ≤ 2 cm), lymph node status, and PR status. (b) Graphical illustration of tamoxifen-related treatment response or follow-up. Time to event (top): time interval between tamoxifen administration and earliest relapse is indicated by green line. Time to follow-up (bottom): time interval between tamoxifen administration and latest follow-up date is indicated by brown line (no tamoxifen-related events observed). (c) Heatmap representation of the pathway activity levels (i.e., NES) and their association with time to tamoxifen-related relapse or follow-up, in the Training cohort. Green line marks the group of patients with tamoxifen-related relapse, sorted from the shortest to the longest time to relapse. Brown line marks the group of patients with follow-up and without disease relapse until the latest follow-up, sorted from the shortest to longest time to follow-up.