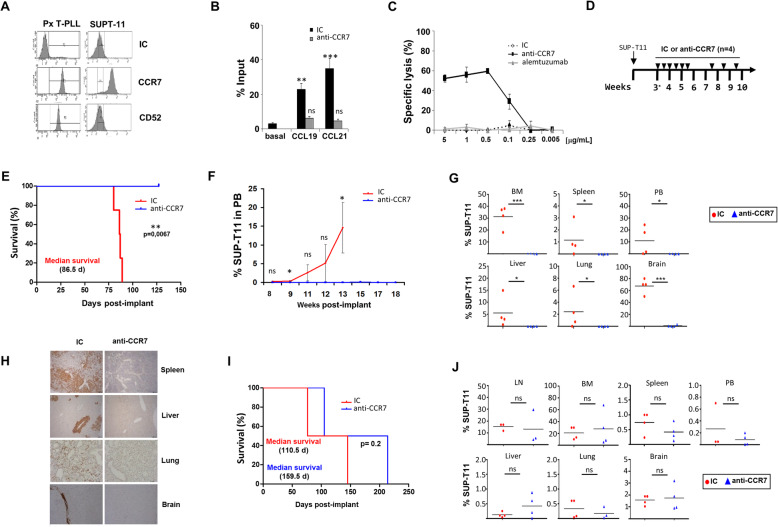
Fig. 4.
Anti-CCR7 is effective as a monotherapy in a novel post-implantation disease model of T-PLL. a SUP-T11 cells show similar CCR7 expression pattern than T-PLL patients. Tumor cells from one representative patient (Px) and from SUP-T11 cell line were stained with anti-CCR7 mAb, anti-CD52 mAb and an irrelevant IC. The graph shows frequency histograms with the pattern and intensity of each surface marker. b Anti-CCR7 mAb blocks migration of SUP-T11 cells. Chemotaxis of SUP-T11 cells induced by CCL19 and CCL21 (1 μg/mL) was assayed in uncoated transwell chambers (n = 3). Where indicated, cells were pre-treated with 10 μg/mL of anti-CCR7 or the respective isotype control, IC. Bars represent mean ± SEM. c Anti-CCR7 mediates CDC on SUP-T11 cells. Cells were incubated with anti-CCR7, anti-CD52 (alemtuzumab) or an IC at the indicated concentrations and then exposed to rabbit complement for 1.5 h (n = 2). Each symbol represents mean ± SD. d Schematic representation of model development and treatment schedule. The novel T-PLL-like systemic model was developed by intravenous inoculation of 5 × 105 SUP-T11 cells into RAG2−/−γc−/− deficient mice. On day 21 (*), when SUP-T11 cells were detected in bone marrow (BM), animals were randomized (n = 4 animals/group). Mice were treated intraperitoneally with 10 mg/kg (~ 200 μg/mouse) of anti-CCR7 mAb or its respective IC twice in weeks 3, 4, 5 and once in weeks 7, 8, 9 (arrows indicate antibody treatment days). e Anti-CCR7 mAb therapy increases survival of SUP-T11-xenografted mice. Survival was estimated and compared by Kaplan-Meier survival curves and log-rank test, respectively. f Anti-CCR7 mAb therapy reduces tumor burden in PB. The percentage of SUP-T11 cells in PB was determined by flow cytometry all over the experiment. Each point represents mean ± SD. g and h Anti-CCR7 mAb therapy impairs infiltration of lymphoid and non-lymphoid organs. g At sacrifice, one million cells from spleen, BM, PB, lung, liver, and brain were harvested and incubated with anti-hCD5 and anti-hCD45 antibodies. The percentage of SUP-T11 cells observed in each tissue is shown. Each symbol represents one individual mouse. Horizontal bars represent mean percentage of SUP-T11 cells of each group. h Immunohistochemistry (IHC) analyses were performed in tissue sections from spleen, liver, brain, and lungs using anti-hCD45 mAb. Representative pictures from both a control mouse and an anti-CCR7 treated mouse are shown. i and j Anti-CCR7 Fab-mediated MOA are insufficient to achieve the maximal therapeutic response. The novel TPLL-like systemic model was developed by intravenous inoculation of 5 × 105 SUP-T11 cells into irradiated NSG deficient mice (see picture d). On day 21, and mimicking model on RAG2−/−γc−/−, animals were randomized (n = 4 animals/group). Mice were treated intraperitoneally with 10 mg/kg (~ 200 μg/mouse) of anti-CCR7 mAb or its respective IC twice in weeks 3, 4, 5 and once in weeks 7, 8, 9 (arrows indicate antibody treatment days). i Fab-mediated MOA in anti-CCR7 therapy induce a non-significant increase in survival. j Fab-mediated MOA in anti-CCR7 therapy do not reduce tumor cell infiltration. At sacrifice, flow cytometry analysis was performed as disclosed in g. Each symbol represents one individual mouse. Horizontal bars represent mean percentage of SUP-T11 cells of each group. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant