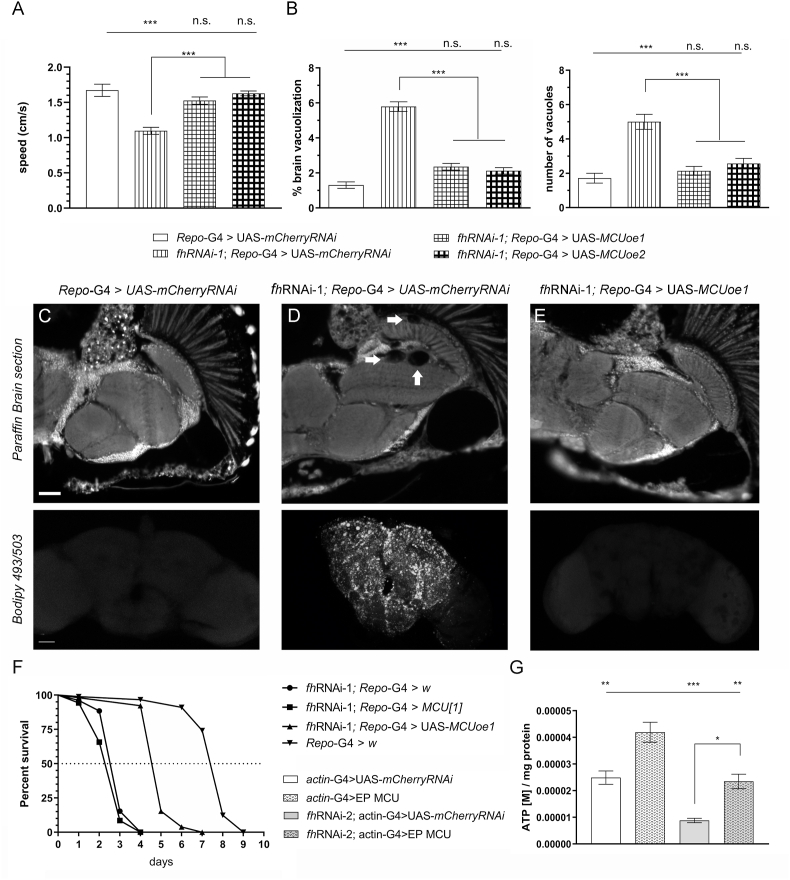
Fig. 6.
Overexpression of Mitochondrial Calcium Uniporter improves several FRDA-like conditions in flies. (A–B) Compared to control flies (Repo-G4>UAS-mCherryRNAi, white column), overexpression of MCU along with frataxin silencing (fhRNAi-1;Repo-G4>UAS-MCUoe1 and fhRNAi-1;Repo-G4>UAS-MCUoe2) successfully restored the locomotor performance (A) as well as the levels of brain degeneration and the number of vacuoles (B) of FRDA flies (fhRNAi-1;Repo-G4>UAS-mCherryRNAi) to normal values. (C–E) Representative paraffin brain sections and Bodipy stainings of controls (C), FRDA flies (D) or overexpression of MCU along with frataxin silencing flies (E). Arrows indicate the presence of the vacuolization in frataxin-deficient brains with normal levels of MCU (D). Importantly, increased expression of MCU in glia was sufficient to reverse the degenerative phenotypes (E, and quantification in B). Complete absence of lipid deposits in controls (C), a strong accumulation in FRDA flies (D) and normal lipid levels upon overexpression of MCU along with frataxin silencing (E). (F) In agreement to our previous results, frataxin silencing in glia shortened longevity under oxidative stress. Importantly, MCU overexpression strongly improved this defect. (G) Similarly, overexpression of MCU (EP-MCU) is sufficient to increase ATP values in controls (actin-G4>EP-MCU) and FRDA flies (fhRNAi-2;actin-G4>EP-MCU). Graphs represent means ± SEM. In A, B and G data was analyzed by One-way ANOVA with post-hoc Tukey Multiple Comparison test. In F, data was analyzed by Log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Scale bars represent 50 μm.