Abstract
Background:
The clinical features of patients with small cell lung cancer (SCLC) and idiopathic pulmonary fibrosis (IPF) have not been fully elucidated.
Patients and methods:
Data on 366 patients with pathologically confirmed SCLC who had been treated with chemotherapy or chemoradiotherapy were retrospectively analyzed to investigate the clinical features of SCLC with IPF.
Results:
A total of 97 out of the 366 patients were diagnosed with interstitial lung disease (ILD), and 75 of them had IPF. For both the limited disease (LD) and extensive disease (ED) stages, the median progression-free survival (PFS) and overall survival (OS) were significantly shorter in the patients with IPF compared with non-ILD patients. A multivariate analysis showed that poor performance status, ED stage, and the presence of IPF were associated with shorter OS. The response rate to first-line therapy was significantly lower in patients with IPF compared with the non-ILD patients. The rate of patients receiving fewer than three cycles of first-line chemotherapy was higher in patients with IPF, which was a factor of poor survival. In LD-stage patients with IPF, chemoradiotherapy was associated with longer PFS and OS compared with chemotherapy only.
Conclusion:
In patients with SCLC, the presence of IPF was associated with a lower response rate as well as shorter PFS and shorter OS. There are some cases that are suitable for chemoradiotherapy, even among patients with IPF.
The reviews of this paper are available via the supplemental material section.
Keywords: acute exacerbation, idiopathic pulmonary fibrosis, interstitial lung disease, response, small cell lung cancer, survival
Introduction
Interstitial lung disease (ILD) is characterized by diffuse pulmonary interstitial abnormalities that often lead to fibrosis.1 Several studies have shown that pre-existing ILD is associated with shorter survival in patients with advanced non-small cell lung cancer (NSCLC)2–4 and small cell lung cancer (SCLC).5–8 Idiopathic pulmonary fibrosis (IPF) is a major chronic fibrosing ILD. Several studies have reported survival in patients with SCLC and IPF.6,9–13 Based on these data sets (n = 10–59), overall survival (OS) was reportedly around 7–16 months. On the other hand, it has been reported that the response rate to first-line chemotherapy was not different between patients with ILD (n = 28) and non-ILD patients.6 However, in NSCLC, a lower disease-control rate for first-line chemotherapy in patients with ILD (n = 53) was reported.4 The response rate in patients with SCLC might also be found to be lower in patients with IPF compared with those without ILD if a greater number of patients is assessed.
No standard chemotherapeutic regimen for patients with SCLC with IPF has been established.10,13 As radiation can produce significant pneumonitis, the correct dose of radiotherapy to the lung in patients with IPF has also not been established.13 In cases of SCLC with underlying IPF, the efficacy and safety of chemotherapy and chemoradiotherapy are largely unknown as well.10–13
As the information regarding patients with SCLC with IPF is limited, we conducted this larger scale study. We have retrospectively compared the efficacy of first-line SCLC therapy and factors related to survival in patients with IPF with those of patients without ILD.
Patients and methods
Patients
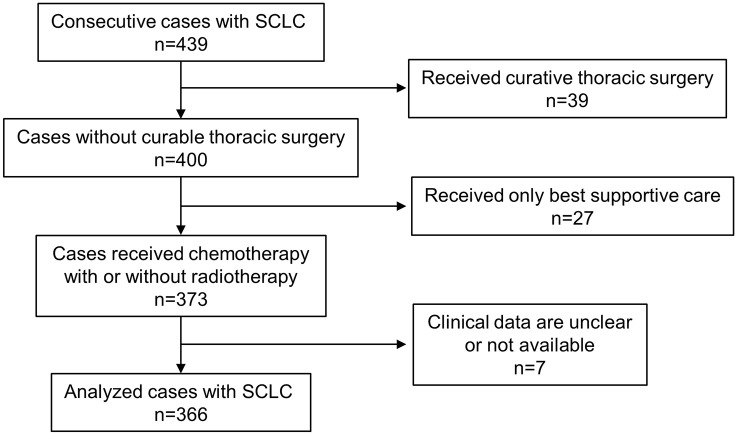
This study was approved by the Institutional Review Board of Aichi Cancer Center Hospital (no. 2017-1-352), Kagawa Prefectural Central Hospital (no. 695), and Kagawa University (no. H29-181). Patients with pathologically confirmed SCLC who presented to any of these hospitals between January 2007 and December 2016 were retrospectively identified, and relevant clinical and laboratory data were collected from their medical records. In all, 439 patients with SCLC were identified, of whom 73 patients were excluded from this study because 39 had received curative thoracic surgery and 27 had received only best supportive care; the clinical data of the 7 other excluded patients were unclear or unavailable. Thus, this study analyzed the cases of 366 patients retrospectively (Figure 1). All patients were diagnosed with SCLC pathologically by transbronchial biopsy (211 cases), endobronchial ultrasound-guided transbronchial needle aspiration (26 cases), computed tomography (CT)-guided biopsy (37 cases), pleural effusion (14 cases), and others (78 cases). In most cases, treatment strategy was discussed by several pulmonologists and the attending physician usually made a final decision on each treatment. Response was assessed according to RECIST, version 1.1.14
Figure 1.
Flowchart for patient selection.
SCLC, small cell lung cancer.
Evaluation of ILD and the diagnosis of IPF
The evaluation of ILD on high-resolution CT was made in accordance with the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society statement.15 The usual interstitial pneumonia (UIP) pattern was defined as having all four of the following features: (a) subpleural basal predominance; (b) reticular abnormality; (c) honeycombing; (d) traction bronchiectasis or bronchiolectasis; and (e) the absence of features listed as alternative diagnosis.15 If honeycombing was absent but other features met the criteria for the UIP pattern, the case was classified as having a probable UIP pattern.15
The clinical diagnosis of IPF was based on the following criteria: UIP or probable UIP patterns on high-resolution CT and exclusion of other known causes of ILD.15,16 No patients in this study underwent a surgical lung biopsy to diagnose IPF. In this study, we did not consider nonfibrotic ILD such as cellular nonspecific interstitial pneumonia (NSIP) and organizing pneumonia to be ILD. Chronic fibrosing ILD, such as fibrotic NSIP, was considered to be non-IPF-ILD.
The diagnosis of acute exacerbation of IPF was made in accordance with the criteria updated in 2016 as follows: (a) acute worsening or development of dyspnea, typically <1 month duration; (b) CT image with new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with the UIP pattern; (c) deterioration not fully explained by cardiac failure or fluid overload.17
Statistical analysis
Progression-free survival (PFS) was defined as the time between the start of chemotherapy and death or the diagnosis of disease progression. OS was defined as the time between the date of diagnosis and the date of death from any cause. PFS and OS curves were constructed by the Kaplan–Meier method, and differences in PFS and OS were compared using the log-rank test for univariate analysis and a Cox proportional hazards model for multivariate analysis. Fisher’s exact test and Student’s t-test were used to analyze patient characteristics. Logistic regression analysis was used to identify factors associated with response rate. Laboratory and pulmonary function data are presented as means ± standard deviation. All statistical analyses were conducted using Ekuseru-Toukei 2015 software (Social Survey Research Information, Tokyo, Japan).
Results
Patient characteristics
A total of 366 patients with pathologically confirmed SCLC were assessed in this study. The relevant characteristics of the patients are shown in Table 1. ILD was identified in 97 patients (26.5%), 75 of whom were diagnosed as having IPF (20.5% of the 366 patients; 34 patients with UIP pattern and 41 patients with probable UIP pattern). The remaining 22 non-IPF-ILD patients included 13 with fibrotic idiopathic NSIP, 1 with Sjögren’s syndrome-related ILD, 1 with polymyositis-related ILD, and 7 with unclassified ILD.
Table 1.
Patient characteristics.
| Characteristic | All patients (n = 366) | Non-ILD (n = 269) | All ILD (n = 97) | p value (versus non-ILD) | IPF (n = 75) | p value (versus non-ILD) | Non-IPF ILD (n = 22) | p value (versus non-ILD) |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| Years (range) | 70 (27–89) | 68 (27–89) | 73 (52–87) | < 0.0001 | 73 (52–87) | < 0.0001 | 73 (55–84) | 0.0168 |
| Gender | ||||||||
| Male | 324 (89%) | 234 (87%) | 90 (93%) | 0.1403 | 71 (95%) | 0.0663 | 19 (86%) | 1.0000 |
| Female | 42 (11%) | 35 (13%) | 7 (7%) | 4 (5%) | 3 (4%) | |||
| Smoking status | ||||||||
| Never | 11 (3%) | 11 (4%) | 0 (0%) | 0.0416 | 0 (0%) | 0.1306 | 0 (0%) | 1.0000 |
| Ever | 355 (97%) | 258 (96%) | 97 (100%) | 75 (100%) | 22 (100%) | |||
| Pack-year, average | 66.8 | 69.9 | 60.1 | 0.2019 | 52.5 | 0.3499 | 49.3 | 0.0287 |
| PS | ||||||||
| 0–1 | 306 (84%) | 224 (83%) | 82 (85%) | 0.8734 | 64 (85%) | 0.7272 | 18 (82%) | 0.7728 |
| 2–4 | 60 (16%) | 45 (17%) | 15 (15%) | 11 (15%) | 4 (18%) | |||
| Stage | ||||||||
| LD | 165 (45%) | 124 (46%) | 41 (42%) | 0.5529 | 26 (35%) | 0.0875 | 15 (68%) | 0.0737 |
| ED | 201 (55%) | 145 (54%) | 56 (58%) | 49 (65%) | 7 (32%) | |||
| Pulmonary function tests | ||||||||
| %VC (%, average) | 89.0 (n = 170) | 88.9 (n = 128) | 89.1 (n = 42) | 0.9644 | 89.6 (n = 30) | 0.8304 | 87.5 (n = 11) | 0.8300 |
| %FVC (%, average) | 84.9 (n = 135) | 84.1 (n = 82) | 86.1 (n = 53) | 0.5250 | 86.9 (n = 38) | 0.4114 | 84.1 (n = 15) | 0.9980 |
| %DLCO (%, average) | 76.7 (n = 136) | 83.9 (n = 97) | 76.4 (n = 30) | 0.1269 | 78.4 (n = 21) | 0.3594 | 71.63 (n = 9) | 0.0906 |
| Blood examination | ||||||||
| KL-6 (U/ml, average) | 651 (n = 58) | 340 (n = 12) | 732 (n = 46) | 0.0003 | 687 (n = 36) | 0.0003 | 895 (n = 10) | 0.1320 |
DLCO, diffusing capacity of lung for carbon monoxide; ED, extensive disease; FVC, forced vital capacity; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LD, limited disease; PS, performance status; VC, vital capacity.
The patients with IPF were significantly older (average 73 years, p < 0.0001) than those without ILD (68 years). All patients with IPF had a smoking history, whereas 11 (4%) of the 269 non-ILD patients never smoked.
Responses to chemotherapy or chemoradiotherapy
All of the patients in this study received active first-line treatment: chemoradiotherapy in 121 patients and chemotherapy in 245 patients (Table 2); 99% of patients received platinum-doublets (cisplatin/carboplatin and etoposide/irinotecan). The number of patients who received carboplatin but not cisplatin was significantly higher in the IPF group compared with the non-ILD group (84% versus 58%, respectively, p < 0.0001). The response rate was significantly lower in patients with IPF than in non-ILD patients (70% versus 86%, respectively, p = 0.0029). The response rate to carboplatin and etoposide was lower in patients with IPF than in non-ILD patients (67% versus 83%, respectively, p = 0.0227).
Table 2.
Response to first-line therapy.
| Characteristics | All patients (n = 366) | Non-ILD (n = 269) | All ILD (n = 97) | p value (versus non-ILD) | IPF (n = 75) | p value (versus non-ILD) | Non-IPF ILD (n = 22) | p value (versus non-ILD) |
|---|---|---|---|---|---|---|---|---|
| First-line therapy | ||||||||
| Chemoradiotherapy (rate in LD) | 121 (73%) | 103 (83%) | 18 (44%) | < 0.0001 | 10 (38%) | < 0.0001 | 8 (53%) | 0.0132 |
| Chemotherapy | 245 | 166 | 79 | 65 | 14 | |||
| First-line chemotherapeutic regimen | ||||||||
| Platinum-doublet | 362 (99%) | 265 (99%) | 96 (99%) | 1.0000 | 75 (100%) | 0.5804 | 21 (95%) | 0.3269 |
| Cisplatin/carboplatin | 131 (36%)/231 (64%) | 113 (42%)/153 (58%) | 18 (19%)/78 (81%) | < 0.0001 | 12 (16%)/63 (84%) | < 0.0001 | 6 (29%)/15 (71%) | 0.2550 |
| Etoposide/irinotecan | 311 (86%)/52 (14%) | 225 (84%)/42 (16%) | 86 (90%)/10 (10%) | 0.2367 | 67 (89%)/8 (11%) | 0.3553 | 19 (90%)/2 (10%) | 0.7516 |
| Others | 4 (1%) | 3 (1%) | 1 (1%) | 0 (0%) | 1 (5%) | |||
| Response to first-line therapy: all/LD/ED stages | ||||||||
| CR | 62/53/9 | 54/45/9 | 8/8/0 | 5/5/0 | 3/3/0 | |||
| PR | 234/97/137 | 173/70/103 | 61/27/34 | 47/17/30 | 14/10/4 | |||
| SD | 26/6/20 | 15/2/13 | 11/4/7 | 10/3/7 | 1/1/0 | |||
| PD | 38/8/30 | 22/6/16 | 16/2/14 | 12/1/11 | 4/1/3 | |||
| NE | 6/1/5 | 5/1/4 | 1/0/1 | 1/0/1 | 0/0/0 | |||
| Response rate | 82/91/74% | 86/93/79% | 72/85/62% | 0.0029/0.1164/0.0170 | 70/85/63% | 0.0029/0.2243/0.0322 | 77/87/57% | 0.3409/0.6280/0.3804 |
| Each chemotherapeutic regimen: CR + PR/SD + PD (response rate) | ||||||||
| Cisplatin + etoposide | 86/10 (90%) | 75/8 (90%) | 11/2 (85%) | 0.6212 | 6/2 (75%) | 0.2124 | 5/0 (100%) | 1.0000 |
| Carboplatin + etoposide | 164/47 (78%) | 115/24 (83%) | 49/23 (68%) | 0.0226 | 39/19 (67%) | 0.0227 | 10/4 (71%) | 0.2888 |
| Cisplatin + irinotecan | 30/3 (91%) | 26/2 (93%) | 4/1 (80%) | 0.3996 | 4/0 (100%) | 1.0000 | 0/1 (0%) | 0.1034 |
| Carboplatin + irinotecan | 10/4 (71%) | 7/3 (70%) | 4/1 (80%) | 1.0000 | 3/1 (75%) | 0.3395 | 1/0 (100%) | 1.0000 |
CR, complete response; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LD, limited disease; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Among the limited disease (LD)-stage patients (n = 165), the number of patients who received chemoradiotherapy was significantly lower in the IPF group compared with the non-ILD group (38% versus 83%, respectively, p < 0.0001). In the extensive disease (ED)-stage patients (n = 201), the response rate was 79% and 63% in the non-ILD patients and patients with IPF, respectively (p = 0.0322).
The number of cycles of first-line chemotherapy received was investigated (Supplemental Table S1). Of the patients with IPF, 39% received three or fewer cycles, which is a significantly higher rate than for non-ILD patients (24%). We next investigated the reasons for the discontinuation of first-line chemotherapy within three cycles. Although there was no significant difference between groups, discontinuation because of adverse events occurred more often in the patients with IPF than in the non-ILD patients (34% and 22%, respectively). The acute exacerbation of IPF occurred in three patients during first-line therapy, resulting in the discontinuation of chemotherapy in all three cases. In all patients who received thoracic radiotherapy, the irradiation dose was 45 Gy in total. In patients with LD stage and ILD, no chemoradiotherapy-related death was observed. One patient experienced acute exacerbation of ILD after irradiation and during a third cycle of cisplatin and etoposide, with recovery treated with corticosteroids.
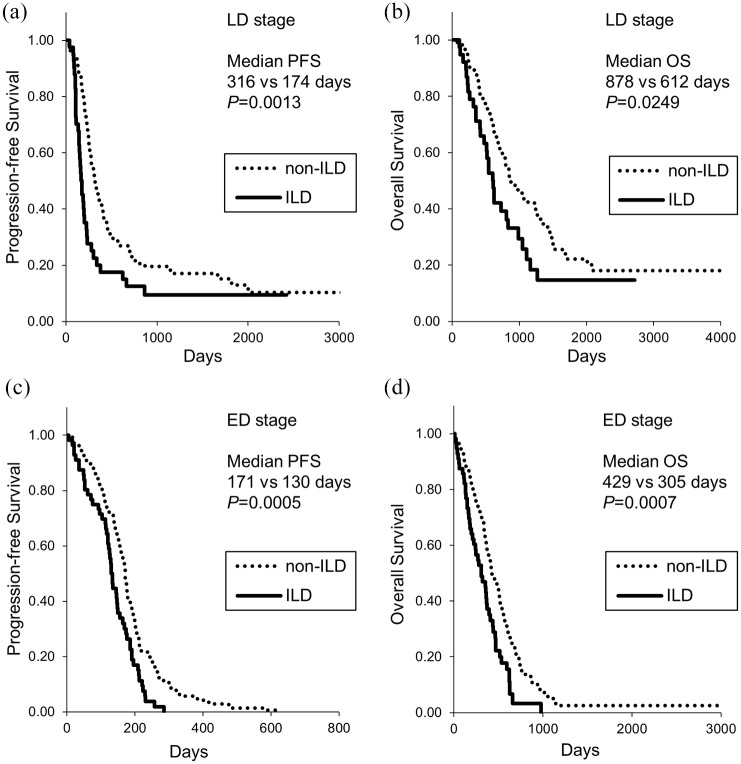
Shorter PFS and OS in patients with ILD
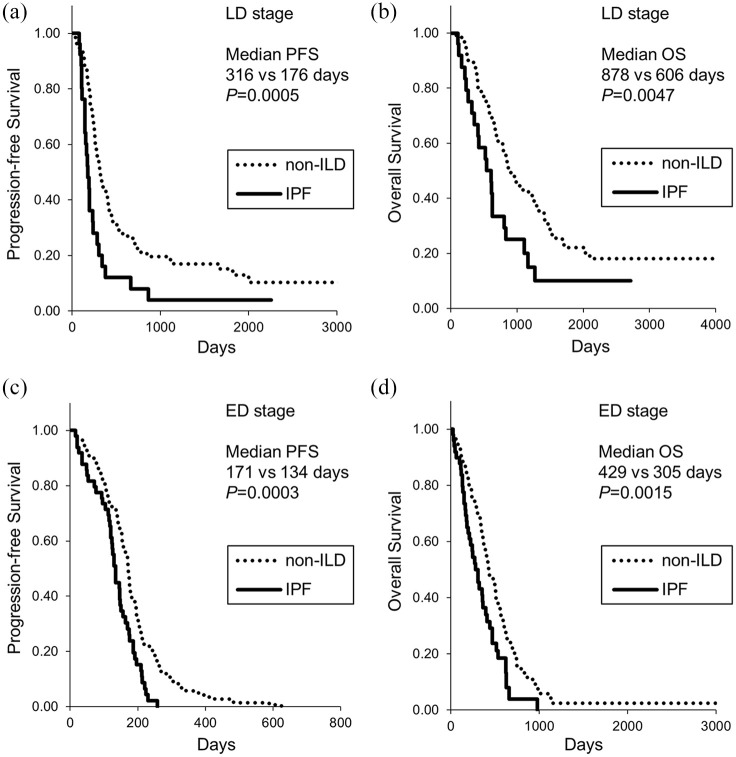
Of 165 LD-stage patients, 32 received prophylactic cranial irradiation and the remaining 133 patients did not. As shown in Figure 2, the patients with ILD showed significantly shorter PFS and OS in the LD stage than non-ILD patients (median PFS, 174 days versus 316 days, respectively, p = 0.0013; median OS, 612 days versus 878 days, respectively, p = 0.0249). In the ED stage, the patients with ILD still showed significantly shorter PFS and OS (median PFS, 130 days versus 171 days, respectively, p = 0.0005; median OS, 305 days versus 429 days, respectively, p = 0.0007). Similarly, the patients with IPF showed shorter PFS compared with non-ILD patients in both LD and ED stages (median PFS of 176 days and 134 days, respectively) (Figure 3). The patients with IPF showed shorter OS compared with non-ILD patients in both LD and ED stages (median OS of 606 days and 305 days, respectively) (Figure 3). There was no difference in PFS and OS between patients with IPF and non-IPF-ILD patients (data not shown), although there was a tendency for shorter OS in patients with IPF (median OS 355 days versus 510 days, p = 0.0508).
Figure 2.
Kaplan–Meier curves of (a, c) PFS and (b, d) OS in patients with small cell lung cancer with ILD. (a) and (b): LD. (c) and (d), ED. ED, extensive disease; ILD, interstitial lung disease; LD, limited disease; OS, overall survival; PFS, progression-free survival.
Figure 3.
Kaplan–Meier curves of (a, c) PFS and (b, d) OS in patients with small cell lung cancer with IPF. (a) and (b): LD. (c) and (d),ED. ED, extensive disease; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LD, limited disease; OS, overall survival; PFS, progression-free survival.
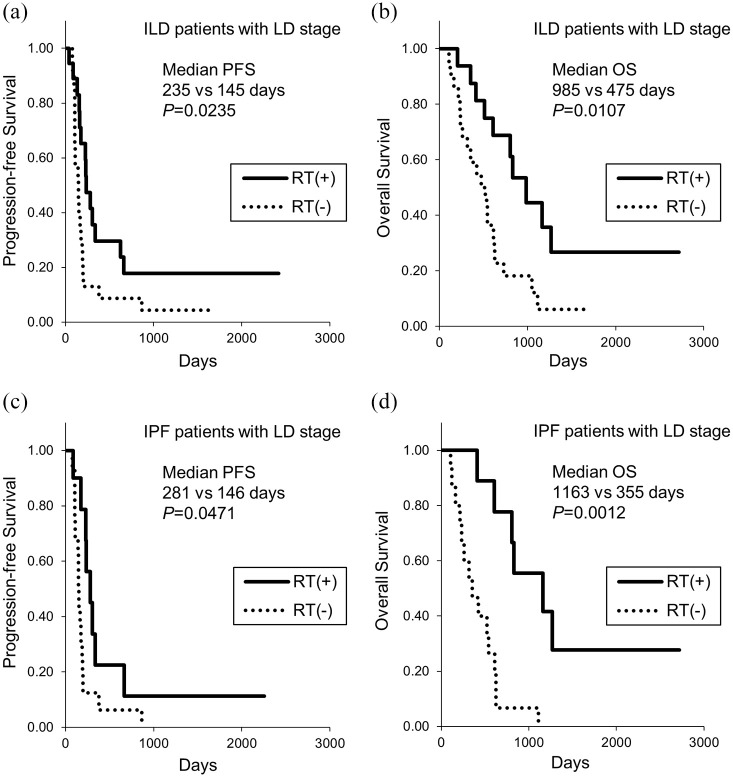
The univariate analysis using the log-rank test identified poor performance status, ED stage, the presence of IPF, and low vital capacity as being associated with poor PFS and OS (Table 3). The multivariate analysis using a Cox proportional hazards model identified ED stage and the presence of IPF as being associated with shorter PFS, and poor performance status, ED stage, and the presence of IPF as being associated with shorter OS (Table 3). To further identify the factors associated with OS in patients with IPF, we analyzed treatment-related factors (Table 4). Multivariate analysis showed that chemotherapy but not chemoradiotherapy in the LD stage, fewer than three cycles of first-line chemotherapy, and no response to first-line therapy were associated with shorter OS (Table 4). In patients with IPF at the LD stage, the PFS as well as OS were significantly longer for patients treated with radiotherapy than without radiotherapy (median PFS, 281 days and 146 days, respectively; median OS, 1163 days and 355 days, respectively) (Figure 4). During all treatment courses, 9 (12%) of 75 patients with IPF had acute exacerbation of IPF. Of them, five patients (56%) died without recovery from the acute exacerbation of IPF.
Table 3.
Risk factors associated with PFS and OS.
| Characteristics | n | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median PFS (days) | Univariate analysis | Multivariate analysis | Median OS (days) | Univariate analysis | Multivariate analysis | ||||
| p value | HR (95% CI) | p value | p value | HR (95% CI) | p value | ||||
| Age, years | |||||||||
| Older (⩾75) | 103 | 191 | 0.2851 | 426 | 0.0527 | ||||
| Younger (<75) | 263 | 194 | 562 | ||||||
| Gender | |||||||||
| Male | 324 | 194 | 0.7272 | 521 | 0.8413 | ||||
| Female | 42 | 186 | 621 | ||||||
| Smoking status | |||||||||
| Ever | 355 | 194 | 0.7429 | 534 | 0.6881 | ||||
| Never | 11 | 181 | 690 | ||||||
| PS | |||||||||
| 2–4 | 60 | 150 | 0.0003 | 1.17 (0.63−2.17) | 0.6196 | 300 | < 0.0001 | 2.81 (1.47−5.40) | 0.0018 |
| 0–1 | 306 | 197 | 584 | ||||||
| Stage | |||||||||
| ED | 201 | 164 | < 0.0001 | 3.68 (2.51−5.39) | < 0.0001 | 395 | < 0.0001 | 3.10 (2.06−4.65) | < 0.0001 |
| LD | 165 | 277 | 842 | ||||||
| ILD | |||||||||
| IPF | 75 | 146 | < 0.0001* | 1.70 (1.12−2.59) | 0.0128 | 355 | < 0.0001* | 1.58 (1.01−2.48) | 0.0443 |
| Non-IPF ILD | 22 | 135 | 0.4257* | 510 | 0.9750* | ||||
| Non-ILD | 269 | 205 | 586 | ||||||
| %VC | |||||||||
| <80% | 58 | 170 | 0.0262 | 1.10 (0.75−1.60) | 0.6321 | 498 | 0.0026 | 1.26 (0.85−1.87) | 0.2513 |
| ⩾80% | 112 | 211 | 671 | ||||||
Versus non-ILD.
CI, confidence interval; ED, extensive disease; HR, hazard ratio; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LD, limited disease; OS, overall survival; PFS, progression-free survival; PS, performance status; VC, vital capacity.
Table 4.
Treatment-related factors associated with OS limited in patients with IPF.
| Characteristics | n | OS | |||
|---|---|---|---|---|---|
| Median OS (days) | Univariate analysis | Multivariate analysis | |||
| p value | HR (95% CI) | p value | |||
| Chemoradiotherapy in LD stage | |||||
| Chemoradiotherapy | 16 | 355 | 0.0012 | 5.02 (1.84−13.68) | 0.0016 |
| Chemotherapy | 10 | 1163 | |||
| Platinum agents | |||||
| Carboplatin | 63 | 318 | 0.0301 | 1.51 (0.70−3.24) | 0.2923 |
| Cisplatin | 12 | 626 | |||
| Another chemotherapeutic agent | |||||
| Irinotecan | 8 | 621 | 0.7525 | ||
| Etoposide | 67 | 355 | |||
| Number of cycles of first-line chemotherapy | |||||
| 1–3 | 29 | 167 | 0.0008 | 2.02 (1.17−3.49) | 0.0120 |
| 4–6 | 46 | 469 | |||
| Acute exacerbation of IPF | |||||
| Yes | 9 | 262 | 0.3942 | ||
| No | 65 | 361 | |||
| Response to first-line therapy | |||||
| SD/PD | 22 | 167 | < 0.0001 | 2.64 (1.46−4.77) | 0.0013 |
| CR/PR | 52 | 471 | |||
CI, confidence interval; CR, complete response; HR, hazard ratio; IPF, idiopathic pulmonary fibrosis; LD, limited disease; OS, overall survival; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 4.
Kaplan–Meier curves of (a, c) PFS and (b, d) OS at LD stage with or without thoracic RT. (a) and (b): patients with ILD. (c) and (d), patients with IPF. ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LD, limited disease; OS, overall survival; PFS, progression-free survival; RT, radiotherapy.
Discussion
In the current study, we investigated a large number of patients with SCLC with ILD (n = 97) and IPF (n = 75). Our analyses demonstrated that: (a) both PFS and OS were shorter in the patients with IPF at both the LD and ED stages; (b) the presence of IPF was associated with a lower response rate to first-line therapy even limited in the ED stage; (c) the rate of patients receiving fewer than three cycles of first-line chemotherapy was higher in patients with IPF, which was a factor in shorter survival; (d) in LD-stage patients with IPF, chemoradiotherapy was associated with longer PFS and OS compared with chemotherapy only.
Several studies have shown poorer prognoses in patients with SCLC with ILD.5–8 In ILD, IPF was reportedly associated with shorter OS.11 The OS was shorter in patients with the advanced gender–age–physiology index.12 Our findings from larger-scale data revealed shorter PFS and OS in patients with ILD and IPF for both LD and ED stages. A more important finding of the present study regards the responses to first-line therapy: the response rate in the patients with IPF was lower than that in the non-ILD patients, even when limited to the ED stage.
There are several possible reasons that could explain the lower response rate and shorter survival of patients with IPF. First, patients with IPF more frequently received fewer cycles of first-line chemotherapy, and fewer cycles of chemotherapy was a poor prognostic factor. One possible reason leading to fewer cycles of chemotherapy in patients with IPF is that these patients may experience adverse events more frequently. Consistent with this, previous studies reported that adverse events occurred more often in patients with NSCLC with ILD than in patients without ILD when they received chemotherapy.4 It was also reported that coexisting ILD was associated with a high risk of developing chemotherapy-induced ILD.18 The second reason to explain the lower response and shorter survival of patients with IPF could be a difference in the rate of cisplatin/carboplatin received as first-line therapy. However, multivariate analysis showed that the platinum agent was not associated with OS in patients with IPF in this study. A meta-analysis of randomized clinical studies in patients with SCLC without ILD showed that there was no difference in OS between cisplatin and carboplatin use.19 Other mechanisms to explain the lower response rate and shorter survival in patients with IPF could include a disturbed drug-delivery system due to the architectural distortion of the lung in IPF, transforming growth factor-beta associated with drug resistance,20 and lung fibroblasts activated in IPF contributing to cancer progression.21,22
The present study showed that undergoing chemoradiotherapy was associated with longer PFS and OS compared with chemotherapy in the LD-stage patients with IPF. The decision to add radiotherapy to chemotherapy was made clinically for each individual case, probably according to several factors. Our findings do not show that radiotherapy is appropriate for all LD-stage patients with IPF. However, our findings clearly show that there are some cases that are suitable for chemoradiotherapy, even among patients with IPF.
We found no significant differences in survivals and other characteristics between the ILD and IPF groups. Therefore, we were unable to find any value to distinguish IPF from ILD in patients with SCLC. However, there was a tendency for a shorter OS in patients with IPF compared with non-ILD-IPF patients (p = 0.0508). In patients with idiopathic interstitial pneumonia (IIP) without lung cancer, survival depends on the type of IIP, and patients with IPF generally have shorter survival than patients with idiopathic NSIP or unclassifiable IIPs.23 When more patients with SCLC are analyzed, some clinical differences between IPF and non-IPF-ILD should be detectable.
The limitations of the present study are as follows. First, this was a retrospective investigation. The therapeutic strategy was determined clinically for each patient. It is difficult to compare the efficacy of each chemotherapeutic regimen in patients with ILD. A second limitation is that ILD classification was determined using high-resolution CT without histopathology. IPF was clinically diagnosed with UIP and probable UIP patterns in this study. The results might change depending on the method of diagnosis of IPF.
In conclusion, this study showed, for the first time, that the presence of IPF was associated with a lower response rate compared with the absence of ILD in patients with SCLC. The patients with IPF had shorter PFS and OS at both the LD and ED stages. The rate of patients receiving fewer than three cycles of first-line chemotherapy was higher in patients with IPF, which was a factor in poor survival. There are some patients at the LD stage who are suitable for chemoradiotherapy rather than chemotherapy even if IPF is present.
Supplemental Material
Supplemental material, Author_Response_1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_2 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_3 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.2 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.3 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplementary_Table_S1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Nobuhiro Kanaji Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing-original draft; Writing–review & editing.
Junichi Shimizu: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Kenichiro Sakai: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Yutaka Ueda: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Hiroshi Miyawaki: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Naohiro Watanabe: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Takehiro Uemura: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Toyoaki Hida: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Takuya Inoue: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Naoki Watanabe: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Yasuhiro Oohara: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Hiroaki Dobashi: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Mikiya Kato: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Norimitsu Kadowaki: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing–review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Nobuhiro Kanaji  https://orcid.org/0000-0001-7365-4927
https://orcid.org/0000-0001-7365-4927
Toyoaki Hida  https://orcid.org/0000-0003-3537-0020
https://orcid.org/0000-0003-3537-0020
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Nobuhiro Kanaji, Department of Internal Medicine, Division of Hematology, Rheumatology and Respiratory Medicine, Faculty of Medicine, Kagawa University, 1750-1 Ikenobe, Miki-cho, Kita-gun, Kagawa 761-0793, Japan.
Junichi Shimizu, Department of Thoracic Oncology, Aichi Cancer Center Hospital, Nagoya, Aichi, Japan.
Kenichiro Sakai, Department of Internal Medicine, Kagawa University, Kita-gun, Kagawa, Japan.
Yutaka Ueda, Department of Respiratory Medicine, Kagawa Prefectural Central Hospital, Takamatsu, Kagawa, Japan.
Hiroshi Miyawaki, Department of Respiratory Medicine, Kagawa Prefectural Central Hospital, Takamatsu, Kagawa, Japan.
Naohiro Watanabe, Department of Thoracic Oncology, Aichi Cancer Center Hospital, Nagoya, Aichi, Japan.
Takehiro Uemura, Department of Thoracic Oncology, Aichi Cancer Center Hospital, Nagoya, Aichi, Japan.
Toyoaki Hida, Department of Thoracic Oncology, Aichi Cancer Center Hospital, Nagoya, Aichi, Japan.
Takuya Inoue, Department of Internal Medicine, Kagawa University, Kita-gun, Kagawa, Japan.
Naoki Watanabe, Department of Internal Medicine, Kagawa University, Kita-gun, Kagawa, Japan.
Yasuhiro Oohara, Department of Internal Medicine, Kagawa University, Kita-gun, Kagawa, Japan.
Hiroaki Dobashi, Department of Internal Medicine, Kagawa University, Kita-gun, Kagawa, Japan.
Mikiya Kato, Department of Internal Medicine, Kagawa University, Kita-gun, Kagawa, Japan.
Norimitsu Kadowaki, Department of Internal Medicine, Kagawa University, Kita-gun, Kagawa, Japan.
References
- 1. Borchers AT, Chang C, Keen CL, et al. Idiopathic pulmonary fibrosis – an epidemiological and pathological review. Clin Rev Allergy Immunol 2011; 40: 117–134. [DOI] [PubMed] [Google Scholar]
- 2. Kinoshita T, Azuma K, Sasada T, et al. Chemotherapy for non-small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol Lett 2012; 4: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishino M, Cardarella S, Dahlberg SE, et al. Interstitial lung abnormalities in treatment-naïve advanced non-small-cell lung cancer patients are associated with shorter survival. Eur J Radiol 2015; 84: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanaji N, Tadokoro A, Kita N, et al. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J Cancer Res Clin Oncol 2016; 142: 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyazaki K, Satoh H, Kurishima K, et al. Interstitial lung disease in patients with small cell lung cancer. Med Oncol 2010; 27: 763–767. [DOI] [PubMed] [Google Scholar]
- 6. Togashi Y, Masago K, Handa T, et al. Prognostic significance of preexisting interstitial lung disease in Japanese patients with small-cell lung cancer. Clin Lung Cancer 2012; 13: 304–311. [DOI] [PubMed] [Google Scholar]
- 7. Kanaji N, Sakai K, Ueda Y, et al. Peripheral-type small cell lung cancer is associated with better survival and higher frequency of interstitial lung disease. Lung Cancer 2017; 108: 126–133. [DOI] [PubMed] [Google Scholar]
- 8. Kanaji N, Tadokoro A, Watanabe N, et al. Association of specific metastatic organs with the prognosis and chemotherapeutic response in patients with advanced lung cancer. Respir Investig 2019; 57: 472–480. [DOI] [PubMed] [Google Scholar]
- 9. Minegishi Y, Takenaka K, Mizutani H, et al. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Intern Med 2009; 48: 665–672. [DOI] [PubMed] [Google Scholar]
- 10. Watanabe N, Taniguchi H, Kondoh Y, et al. Chemotherapy for extensive-stage small-cell lung cancer with idiopathic pulmonary fibrosis. Int J Clin Oncol 2014; 19: 260–265. [DOI] [PubMed] [Google Scholar]
- 11. Koyama N, Iwai Y, Nagai Y, et al. Idiopathic pulmonary fibrosis in small cell lung cancer as a predictive factor for poor clinical outcome and risk of its exacerbation. PLoS One 2019; 14: e0221718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song MJ, Lim SY, Park JS, et al. Prognosis of small cell lung cancer with idiopathic pulmonary fibrosis: assessment according to GAP stage. J Oncol 2019; 2019: 5437390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tzouvelekis A, Karampitsakos T, Gomatou G, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. A retrospective multicenter study in Greece. Pulm Pharmacol Ther 2020; 60: 101880. [DOI] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 15. Raghu G, Remy-Jardin M, Myers JL, et al. ; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. [DOI] [PubMed] [Google Scholar]
- 16. Richeldi L, du Bois RM, Raghu G, et al. ; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 17. Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. [DOI] [PubMed] [Google Scholar]
- 18. Sakurada T, Kakiuchi S, Tajima S, et al. Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother 2015; 49: 398–404. [DOI] [PubMed] [Google Scholar]
- 19. Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012; 30: 1692–1698. [DOI] [PubMed] [Google Scholar]
- 20. Yokokura S, Kanaji N, Tadokoro A, et al. Confluence-dependent resistance to cisplatin in lung cancer cells is regulated by transforming growth factor-beta. Exp Lung Res 2016; 42: 175–181. [DOI] [PubMed] [Google Scholar]
- 21. Kanaji N, Yokohira M, Nakano-Narusawa Y, et al. Hepatocyte growth factor produced in lung fibroblasts enhances non-small cell lung cancer cell survival and tumor progression. Respir Res 2017; 18: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanaji N, Yokohira M, Watanabe N, et al. Transforming growth factor-beta produced by non-small cell lung cancer cells contributes to lung fibroblast contractile phenotype. Anticancer Res 2018; 38: 2007–2014. [DOI] [PubMed] [Google Scholar]
- 23. Fujisawa T, Mori K, Mikamo M, et al. Nationwide cloud-based integrated database of idiopathic interstitial pneumonias for multidisciplinary discussion. Eur Respir J 2019; 53: 1802243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_2 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_3 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.2 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.3 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplementary_Table_S1 for Clinical features of patients with small cell lung cancer and idiopathic pulmonary fibrosis treated with chemotherapy or chemoradiotherapy by Nobuhiro Kanaji, Junichi Shimizu, Kenichiro Sakai, Yutaka Ueda, Hiroshi Miyawaki, Naohiro Watanabe, Takehiro Uemura, Toyoaki Hida, Takuya Inoue, Naoki Watanabe, Yasuhiro Oohara, Hiroaki Dobashi, Mikiya Kato and Norimitsu Kadowaki in Therapeutic Advances in Respiratory Disease