Abstract
Fetal growth restriction (FGR) is a major cause of perinatal morbidity and mortality. Identifying which pregnancies are at risk of FGR facilitates enhanced surveillance and early delivery before fetal demise can ensue. However, existing risk stratification strategies yield an unacceptably low detection rate. A robust and reliable first trimester screening test for FGR would not only enable high-risk women to be appropriately monitored but would facilitate future trials for possible interventions to enhance fetal growth. Both the volume and vascularity of the first trimester placenta has been demonstrated to be linked to adverse pregnancy outcomes including FGR and pre-eclampsia. The investigation of novel ultrasound markers for FGR are discussed along with the development of methods for fully automatic placental volume estimation which has the potential for use as part of a multi-variable population-based screening test.
Keywords: Placental volume, fetal growth restriction, stillbirth, screening, ultrasound, power Doppler
Introduction
Birth weight is dependent upon numerous factors including gestational age at the time of delivery [1]; maternal characteristics [1–3]; and placental volume, vasculature and function [4]. FGR is the term used to describe babies that do not achieve their inherent growth potential, and this term is often used synonymously with ‘small-for-gestational-age’ (SGA) although the two are distinctly different. SGA is defined as a birth weight <10th centile on either customised or population based growth charts, and is frequently used as a surrogate marker for FGR in the research literature. This is problematic because by definition SGA can include babies that are constitutionally small and yet are achieving their growth potential, and may also exclude some that do not achieve their growth potential but remain above the 10th centile. Nevertheless, SGA, especially SGA defined using customised centiles (cSGA), has been shown to be associated with adverse perinatal outcomes including stillbirth and therefore cSGA has been used as a surrogate outcome for FGR in most of the studies discussed in this review.
Despite improvements in healthcare, the incidence of stillbirth in the United Kingdom (UK) has remained relatively static over the last two decades [5] at around 44/10,000 [6, 7]. As the majority of FGR is not diagnosed prior to birth [8, 9], early identification triggering appropriate management may prove to be the key to reducing this dismal figure.
Risk stratification for growth restriction in the UK is generally based on an assessment of maternal characteristics and history at the booking appointment with the community midwife [10]. If serum pregnancy-associated plasma protein-A (PAPP-A) has been measured as part of the trisomy screening protocol then this may be taken into account too as low PAPP-A at 11–14 weeks is associated with an increased risk of SGA [11]. The usefulness of this marker is limited by the uptake of trisomy screening, which is around 66% in the UK [12]. Some women may be offered uterine artery Doppler studies at 20–24 weeks’ gestation, but this is of limited usefulness in the general population [13]. Pregnancies classified as lower risk are then monitored with serial symphysio-fundal height measurements. This technique performs poorly, with detection rates as low as only 14% in some studies [14]. The increasing obesity epidemic in the obstetric population is also affecting the utility of this method.
The diagnosis of FGR could be improved with the introduction of an effective first trimester screening test. This would likely reduce perinatal morbidity and mortality by facilitating enhanced surveillance of higher risk pregnancies, with intervention when necessary. The aetiology of FGR is complex, but utero-placental insufficiency is implicated in many of the cases [15]. The placenta is easily identifiable with ultrasonography from 11–14 weeks’ gestation, along with much of it’s vasculature [16], and thus placenta-based ultrasound markers are an attractive target for the development of a screening test. Such a test would ideally be largely automated in order to allow rapid and simple population-wide implementation, and would be combined with maternal characteristics and biochemical markers such as PAPP-A to form a multivariable test for FGR analogous to the ‘combined test’ currently offered for fetal aneuploidies.
If FGR could be reliably predicted this would not only allow increased fetal monitoring but would create an opportunity to investigate potential treatments. The recent ASPRE trial demonstrated that administration of aspirin to a population identified as high risk using a first trimester screening test reduced the incidence of pre-eclampsia compared with placebo [17], but was underpowered to detect an effect on FGR/SGA and stillbirth. Gene therapy [18] has also been suggested as a possible candidate for treatment of FGR and has shown promise in animal models. Thus, if high-risk pregnancies can be appropriately identified, there is hope that growth restriction and it’s associated morbidity and mortality could one day be reduced if not prevented.
Placental volume
It has long been understood that the weight of the placenta at delivery is positively correlated to birth weight [19, 20]. Since the 1970s it has also been possible to assess the size of the placenta in early pregnancy using two-dimensional (2-D) ultrasonography [21, 22], and it was suggested as early as 1981 that placental size might be predictive of growth restriction [22]. However, difficulty in delineating the required sonographic planes limited the usefulness of this technique. The advent of three-dimensional (3-D) ultrasound in the 1990s improved the accuracy with which measurements could be taken and this generated renewed research interest [23–25]. It has now been shown that low placental volume (PlaV) at 11–13 weeks is associated with SGA/FGR [23, 26–28] and pre-eclampsia [28, 29], and that this is independent of PAPP-A and nuchal translucency (NT) measurements [27]. It has also been shown that the predictive accuracy for SGA can be improved by considering PlaV in combination with other known risk factors – including maternal characteristics, PAPP-A levels and placental growth factor levels [30].
A number of similar methods have been proposed for 2-D assessment of PlaV, in which measurements from multiple 2-D scans in different planes are used to construct an estimate of 3-D placental volume [21, 31–33]. These methods are relatively simple and have the advantage of not requiring expensive 3-D ultrasound technology proprietary software, both of which may not be readily available in resource poor settings. However, assessments of PlaV generated using 2-D ultrasound correlate poorly with validated 3-D ultrasound techniques and are probably less reliable with certain placental shapes, particularly in the first trimester [34]. This may be because the choice of analysis plane is very operator dependent. The time-consuming nature of this method, it’s lack of inter-operator reliability, and it’s unfavourable comparison with other methods means that measurement of PlaV with 2-D ultrasound is not a good candidate for a screening test.
Segmentation of the placenta
Segmentation in any medical imaging modality involves demarcation of the borders of the target organ to identify it within the overall image. The ease of this process depends on the imaging modality used and the clarity of the borders of the target organ. Segmentation using ultrasound presents a distinct challenge, as the borders of the placenta can be very difficult to differentiate from the underlying myometrium in the first trimester. The degree of difficulty varies depending upon placental position (posterior placentas can be affected by artefacts from the overlying fetus), the degree of attenuation of the ultrasound beam (usually by adipose tissue) or the presence of placental lesions.
Manual segmentation
The gold standard for 3-D placental segmentation is manual delineation of the placenta. This technique involves breaking the 3-D image down into 2-D slices. An operator then manually highlights the boundaries of the placenta on each slice. The accuracy of the segmentation relies on the experience of the operator with placental ultrasound images. Even in the best hands though, in early pregnancy there can be very poor sonographic differentiation between the placenta and myometrium, which leads to difficulty determining the true placental border [35]. Consequently, manual delineation of the placenta is highly operator dependent and time-consuming, making this method impractical for use as a screening test.
Semi-automated segmentation using Virtual Organ Computer-aided AnaLysis (VOCAL™)
In this technique a 3-D image of the placenta is captured and a number of 2-D segments are then generated by rotation around a user-defined axis. The number of images depends on the angle of rotation (12°, 15°, 18° or 30°). The placental contour must then be defined manually in each section. The VOCAL™ software then generates a 3-D volume by interpolation around the axis. This method is purely geometric and consequently estimation of volume in an irregularly shaped organ may be more prone to error [35], this is important as it has been demonstrated that the shape of the placenta in the first trimester is not always regular [34].
Assessment of PlaV using this commercially available tool is still time consuming and requires considerable training and is therefore not suitable for use as a screening tool. Further, VOCAL™ is proprietary software and requires that data be obtained using manufacturer-specific ultrasound machines. There is also some uncertainty regarding the repeatability and reproducibility of volume results produced using this method, with certain iterations of the VOCAL™ software producing less reliable results [36].
Semi-automated segmentation using the random walker algorithm
The random walker algorithm allows for semi-automated image segmentation [37], which can produce a 3-D outline of the placenta in considerably less time and a less operator dependent manner. This technique involves labelling (seeding) a small number of pixels/voxels as belonging to either the placenta or the surrounding tissue. Specialised software then calculates the probability that any given pixel/voxel belongs to the area of interest, in this case the placenta, and the borders of the placenta are therefore identified within the image.
The random walker algorithm overcomes the limitations of VOCAL™ with respect to an irregularly shaped placenta, is less likely to over-estimated placental volume compared with 2-D techniques and its initialisation is significantly faster compared with VOCAL (p = <0.001) [34, 35]. Despite faster volume acquisition, the random walker method is comparable to manual segmentation with respect to reproducibility and outperforms VOCAL™ in terms of inter-observer reliability [35].
Use of the random walker method requires relatively little training, demonstrates good intra and inter-observer repeatability and is also non-proprietary, thus some of the barriers to use as a screening test are reduced. However, this method can still be affected by human error [35, 38].
Automated segmentation using deep learning
Deep learning is part of the broader field of machine learning, a specialised area of computer science that focuses the use of statistical techniques to allow computer systems to ‘learn’. In this context, ‘learning’ means using data to progressively improve performance on a specific task without being explicitly programmed to do so. Computers capable of deep learning make use of artificial neural networks, systems designed to emulate some of the functionality of biological neural networks, to perform tasks by ‘learning’ from demonstrated examples of the task performed correctly, without requiring task-specific programming.
It has been shown that artificial neural networks can be trained using ‘ground-truth’ 3-D placental images segmented with the random walker algorithm and quality assured by clinicians (information provided by direct observation used to ‘teach’ the skill in question) [39, 40]. The largest ‘ground truth’ data set, the size of which correlates with the performance of the tool, was used with the novel neural network, OxNNet, and resulted in a mean dice similarity coefficient (an index representing similarity between predicted and manually estimated data) of 0.84 (range 0.72–0.92) [40] (see Figure 1). The placental volume estimates generated with this method perform well in the prediction of SGA when compared with those generated by the random walker algorithm, and outperform PlaV estimates generated with VOCAL™ [35, 38, 41] (see Figure 2).
Figure 1.
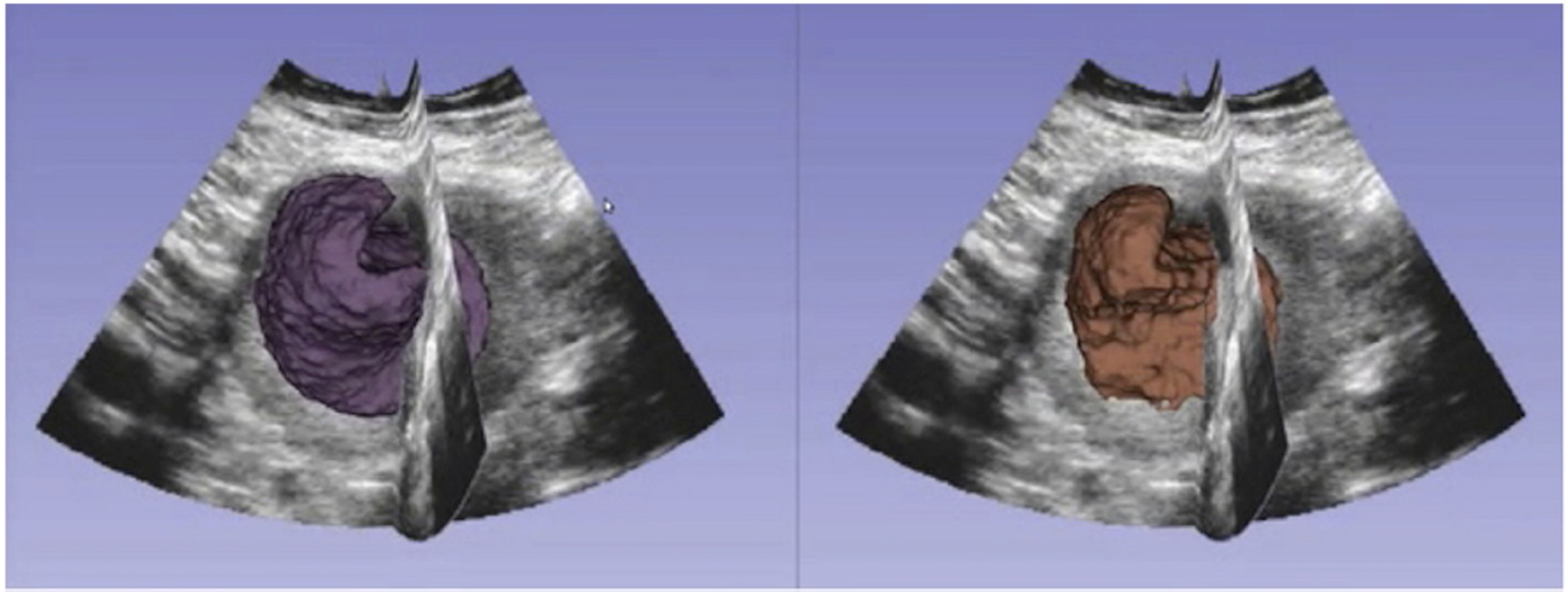
An example of the same placenta segmented using the random walker algorithm (right) and the fully automated method OxNNet (left).
Figure 2.
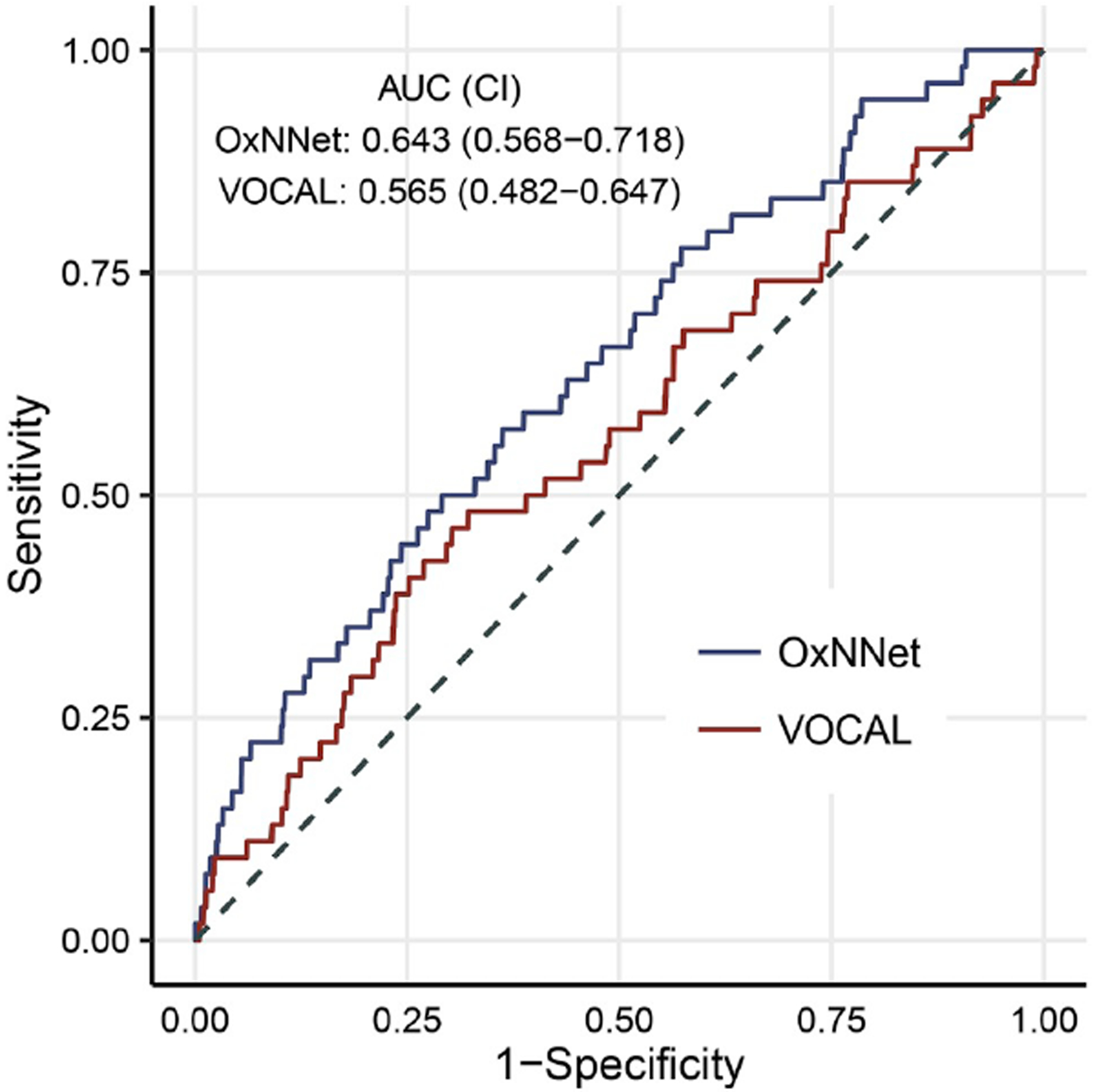
ROC curve comparing placental volumes derived using OxNNet and VOCAL™ (from Looney et al [41]).
As OxNNet provides a fully automated and fast method for PlaV estimation this has the distinct potential to be part of a population-based screening test [40]. Further research and testing is needed, but as this tool can be applied to very large data sets, investigation of the relationship between first trimester placental volume and shape with rare adverse outcomes such as stillbirth is now a possibility for future studies.
Standardised placental volume and its utility
Placental volume increases with gestational age and therefore measurements taken at different gestational ages between 11 and 13 weeks must take this physiological variation into account. This can be achieved using a novel index, the standardised placental volume (sPlaV), calculated as PlaV1/3 (mm3) divided by CRL (mm) [42]. This produces dimensionless and normally distributed results making it more mathematically sound than the placental quotient from which it was derived, the latter being calculated as crown rump length (mm) divided by PlaV (mL or mm3) and having a unit of mm−2 [43].
A pilot study of 143 women has shown that sPlaV, derived using the random walker algorithm, is significantly different in cSGA compared with appropriate for gestational age (AGA) pregnancies (p = <0.001) and that combining sPlaV with PAPP-A and NT produces a predictive model for cSGA. Receiver operating characteristic (ROC) curves for prediction of cSGA in an unselected population using sPlaV alone give an area under the curve of 0.77 (0.66 to 0.87), or 0.80 (0.69 to 0.92) when used in combination with PAPP-A and NT [42]. Further investigation with a larger cohort is required, but this is certainly demonstrates promise as a potential future screening test.
Markers within the utero-placental vasculature
It has been known since the 1980s that high resistance changes in the uterine artery (UtA) Doppler waveform were correlated with FGR/SGA and pre-eclampsia, this has been studied extensively since [44]. There is also histological evidence that FGR/SGA and PET are associated with disordered remodelling of the spiral arteries [45–47]. For many years it was postulated that downstream deficiencies in spiral artery remodelling produced increased resistance to blood flow, manifesting as in increase in upstream vessel impedance. However, the observed changes in uterine artery Doppler indices cannot be as a direct result of inadequate spiral artery remodelling as the waveform does not change immediately after delivery of the placenta, when the spiral arteries are completely closed [48]. Further, it has been shown that normal UtA waveforms occur in cases of abdominal pregnancy where the spiral arteries are not exposed to any trophoblast [49]. This is most likely because the maternal blood is redirected through an arterio-venous anastomotic network in the myometrium, which acts as a buffer. This has been modelled mathematically demonstrating that the vessels most likely to be directly responsible for the changes seen in the uterine artery waveform are the radial arteries [50] and not the spiral arteries. If the association between deficient spiral artery remodelling and increased UtA impedance is not causative, it is understandable why the uterine artery waveform may not be an ideal screening tool as it is probably not sensitive enough to small changes in the vasculature at the utero-placental interface. Therefore, the actual vessels implicated in poor placentation (the spiral arteries) need to be examined directly.
Spiral artery jets
Numerous attempts have been made to directly image the spiral arteries within the myometrium but this has generated conflicting data [51–56]. Early attempts at identification of the spiral arteries were based on their approximate location in the placental bed and their distinctive bidirectional waveform. Unfortunately this method is flawed as lack of defined anatomical landmarks means it is impossible to measure vessels consistently at the same point, angle correction is not appropriate due to the tortuous nature of the spiral artery, and seeking the classic waveform might lead to preferential selection of normal vessels for measurement [16]. However, using simple greyscale ultrasound it is possible to identify the utero-placental interface, the point at which spiral arteries discharge into the intervillous space. Here, a unidirectional stream of blood is discharged from the terminal end of the spiral artery, such streams are termed spiral artery ‘jets’. Jets can be identified as demonstrating unidirectional flow using colour Doppler, and pulsed-wave Doppler can then be used to assess the haemodynamics and waveform of the jet itself [16]. The pulsatility index (PI) and resistance index (RI) of the spiral artery jets have been shown to decrease with advancing gestational age, likely due to the progressive remodelling of the vessel. As disordered remodelling is known to correlate with SGA, this could be a potential screening target. Indeed, pilot data demonstrates that the Jet PI is significantly altered in cSGA pregnancies, an effect that was not seen in the UtA Doppler indices [16]. Further, the jet mouth opening has been shown to increase between 11–13 weeks and 34 weeks, and cSGA pregnancies show a trend towards a smaller change in mouth size (p=0.05) [57]. Jet Doppler indices do show promise, but visualisation of individual vessels and jets is still problematically time consuming and requires considerable training. However, the advent of 3-D power Doppler has provided a means of assessing blood flow across the entire utero-placental interface, and thus this principle has continued to attract research interest.
The use of power Doppler imaging at the utero-placental interface
Power Doppler provides a visual representation of the concentration of moving red blood cells by using the amplitude of Doppler signal to detect moving matter. It is independent of velocity and direction of flow, which is helpful in the imaging of tortuous vessels. Power Doppler is also virtually angle independent which allows better detection of low-flow velocities compared with colour Doppler [58–60].
Power Doppler can be used with both 2-D and 3-D imaging techniques to produce indices of vascularity and perfusion, giving 3-D power Doppler potential to capture information about all of the spiral arteries supplying the placental bed in one go, rather than measuring one at a time as in the case of spiral artery jets. Power Doppler is, however, influenced by body habitus and machine settings, gain in particular [61]. Standardisation is therefore essential in order to allow interpretation of any results obtained.
Standardisation of 2-D power Doppler imaging is possible using the validated technique of fractional moving blood volume (FMBV), in which a large blood vessel is identified and used as a point of reference. Measurements of other vessels are then compared with this ‘reference vessel’ (a point of 100% vascular amplitude) [62] on the assumption that any beam path factors will alter signals from the area of interest and the standardisation point to the same degree [63].
A novel 3-D image-processing technique has recently been developed which uses the raw data exported directly from the ultrasound machine and allows the rapid calculation of FMBV [64]. This technique can facilitate standardized measurement of the vasculature of the entire utero-placental interface in the first trimester (FMBV-UPI). It has so far been used to assess the vascularity of the whole utero-placental interface (FMBV-UPI) as well as that of the intervillous space (FMBV-IVS) [64].
Using this technique, a small pilot study has measured the vascularity of the utero-placental interface and intervillous space in the first trimester [65]. This demonstrated that pregnancies destined to develop pre-eclampsia have small hypovascular placentas, whereas those destined to result in normotensive cSGA have small placentas but with normal vascularity.
Conclusion
It is now possible to automatically measure the volume of the first trimester placenta in real-time using a validated computerised tool, OxNNet. The placental volumes generated outperform the commercially available tool VOCAL™ in the prediction of cSGA. Pilot data suggests that both placental volume and vascularity measurements have utility in the prediction of pre-eclampsia and cSGA. If complete automation of the vascularity index 3-D FMBV can be achieved, this has real potential to be another factor in a possible multi-variable a screening test for both conditions. Larger scale data collection is currently underway to further test the findings of these pilot studies and work continues to improve the fully automated process for segmentation of the placenta from 3-D US. Thus, the development of a fully automated screening test for growth restriction incorporating placental ultrasound markers in the first trimester is a realistic and exciting prospect for the near future.
Figure 3.
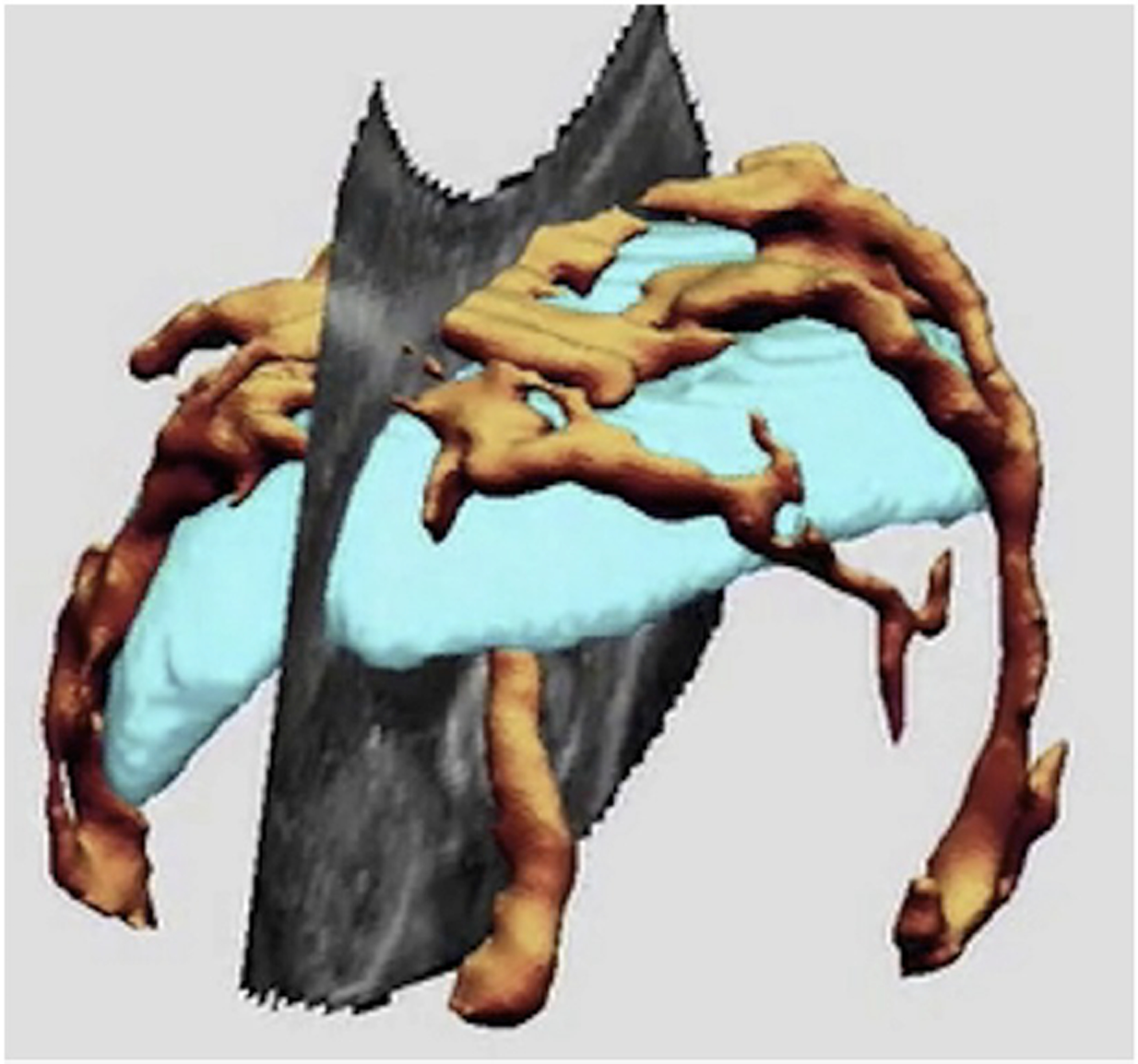
3-D ultrasound image of with placenta, with the placenta segmented out In pale blue and vascularity recorded with power Doppler ultrasound in orange.
Table 1.
Comparison of methods of placental segmentation
| Method | Advantages | Disadvantages |
|---|---|---|
| Manual segmentation | Gold standard for delineation of the placenta. | Very time consuming Highly operator dependent [35]. |
| VOCAL | Faster than manual segmentation. | Still too time consuming for use as a screening tool Proprietary software requiring the use of manufacturer specific ultrasound machines Prone to error with irregularly shaped organs such as the placenta[35]. Reproducibility and reliability of results may be limited [36]. |
| Random walker | Good intra and inter-observer repeatability [35]. Non-proprietary. Requires relatively little training. Faster and less operator dependent [37]. |
Able to deal with irregularly shaped placentae [38]. Still prone to some degree of human error [38]. |
| Deep learning | Calculated PlaVs perform well compared with random walker and outperform VOCAL in the prediction of SGA [35, 38, 41]. Fully automated and fast [40]. |
Dependent of quality of ground truth data set[39, 40]. |
Aim
To review novel automated techniques for ultrasound measurement of placental volume and utero-placental perfusion, and to illustrate how these might have utility in the development of a screening test for fetal growth restriction.
Acknowledgments
The authors thank Dr Padraig Looney, Dr Gordon Stevenson and Prof. J. Alison Noble for their pioneering placental image analysis that made much of this work possible. We gratefully acknowledge the support of NVIDIA Corporation who donated the Tesla GTX Titan X GPU used for some of the image analysis research. Sally Collins is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Human Placenta Project of the National Institutes of Health under award number UO1-HD087209. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interests
The authors have nothing to declare.
References
- 1.Poon LC, et al. , Reference range of birth weight with gestation and first-trimester prediction of small-for-gestation neonates. Prenat Diagn, 2011. 31(1): p. 58–65. [DOI] [PubMed] [Google Scholar]
- 2.Wen SW, et al. , Smoking, maternal age, fetal growth, and gestational age at delivery. Am J Obstet Gynecol, 1990. 162(1): p. 53–8. [DOI] [PubMed] [Google Scholar]
- 3.Clausson B, Cnattingius S, and Axelsson O, Preterm and term births of small for gestational age infants: a population-based study of risk factors among nulliparous women. Br J Obstet Gynaecol, 1998. 105(9): p. 1011–7. [DOI] [PubMed] [Google Scholar]
- 4.Salafia CM, Charles AK, and Maas EM, Placenta and fetal growth restriction. Clin Obstet Gynecol, 2006. 49(2): p. 236–56. [DOI] [PubMed] [Google Scholar]
- 5.Seaton SE, et al. , Socioeconomic inequalities in the rate of stillbirths by cause: a population-based study. BMJ Open, 2012. 2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clausson B, et al. , Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG, 2001. 108(8): p. 830–4. [DOI] [PubMed] [Google Scholar]
- 7.Flenady V, et al. , Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet, 2011. 377(9774): p. 1331–40. [DOI] [PubMed] [Google Scholar]
- 8.Hepburn M and Rosenberg K, An audit of the detection and management of small-for-gestational age babies. Br J Obstet Gynaecol, 1986. 93(3): p. 212–6. [DOI] [PubMed] [Google Scholar]
- 9.Kean L and Liu D, Antenatal care as a screening tool for the detection of small for gestational age babies in the low risk population. Journal of Obstetrics and Gynaecology, 1996. 16(2): p. 77–82. [Google Scholar]
- 10.RCOG, The investigation and management of the small-for-gestational-age fetes (Green-top guideline No. 31) 2014, Royal College of Obstetricians and Gynaecologists. [Google Scholar]
- 11.Cowans N and Spencer K, <p class=“MsoNormal” style=“margin-top:6.0pt;margin-right:0cm;margin-bottom:6.0pt;margin-left:0cm;line-height:15.0pt;mso-outline-level:1”>First-trimesterADAM12 and PAPP-A as markers for intrauterine fetal growth restriction throughtheir roles in the insulin-like growth factor system. Prenat diagn, 2007. 27: p. 264–271. [DOI] [PubMed] [Google Scholar]
- 12.Chitty LS, et al. , Uptake, outcomes, and costs of implementing non-invasive prenatal testing for Down’s syndrome into NHS maternity care: prospective cohort study in eight diverse maternity units. BMJ, 2016. 354: p. i3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cnossen JS, et al. , Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ, 2008. 178(6): p. 701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Backe B and Nakling J, Effectiveness of antenatal care: a population based study. Br J Obstet Gynaecol, 1993. 100(8): p. 727–32. [DOI] [PubMed] [Google Scholar]
- 15.Sankaran S and Kyle PM, Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol, 2009. 23(6): p. 765–77. [DOI] [PubMed] [Google Scholar]
- 16.Collins S, et al. , Measurement of spiral artery jets: general principles and differences observed in small-for-gestational-age pregnancies. Ultrasound obstet gynecol, 2012. 40(2): p. 171–178. [DOI] [PubMed] [Google Scholar]
- 17.Rolnik DL, et al. , Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med, 2017. 377(7): p. 613–622. [DOI] [PubMed] [Google Scholar]
- 18.Jones HNC, Timothy Habli, Mounira, Adenoviral-Mediated Placental Gene Transfer of IGF-1 Corrects Placental Insufficiency via Enhanced Placental Glucose Transport Mechanisms. PLoS ONE, 2013. 8(9): p. e74632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LITTLE WA, The significance of placental/fetal weight ratios. Am J Obstet Gynecol, 1960. 79: p. 134–7. [DOI] [PubMed] [Google Scholar]
- 20.SINCLAIR JG, Placental-fetal weight ratios. Tex Rep Biol Med, 1948. 6(2): p. 168–75. [PubMed] [Google Scholar]
- 21.Bleker OP, et al. , The volumetric growth of the human placenta: a longitudinal ultrasonic study. Am J Obstet Gynecol, 1977. 127(6): p. 657–61. [DOI] [PubMed] [Google Scholar]
- 22.Jones TB, Price RR, and Gibbs SJ, Volumetric determination of placental and uterine growth relationships from B-mode ultrasound by serial area-volume determinations. Invest Radiol, 1981. 16(2): p. 101–6. [DOI] [PubMed] [Google Scholar]
- 23.Hafner E, et al. , Correlation of first trimester placental volume and second trimester uterine artery Doppler flow. Placenta, 2001. 22(8–9): p. 729–34. [DOI] [PubMed] [Google Scholar]
- 24.Riccabona M, et al. , Distance and volume measurement using three-dimensional ultrasonography. J Ultrasound Med, 1995. 14(12): p. 881–6. [DOI] [PubMed] [Google Scholar]
- 25.Riccabona M, Nelson TR, and Pretorius DH, Three-dimensional ultrasound: accuracy of distance and volume measurements. Ultrasound Obstet Gynecol, 1996. 7(6): p. 429–34. [DOI] [PubMed] [Google Scholar]
- 26.Plasencia W, et al. , Placental volume at 11–13 weeks’ gestation in the prediction of birth weight percentile. Fetal Diagn Ther, 2011. 30(1): p. 23–8. [DOI] [PubMed] [Google Scholar]
- 27.Law LW, et al. , Which ultrasound or biochemical markers are independent predictors of small‐for‐gestational age? Ultrasound Obstet Gynecol, 2009(34): p. 283–287. [DOI] [PubMed] [Google Scholar]
- 28.Hafner E, et al. , Comparison between three-dimensional placental volume at 12 weeks and uterine artery impedance/notching at 22 weeks in screening for pregnancy-induced hypertension, pre-eclampsia and fetal growth restriction in a low-risk population. Ultrasound Obstet Gynecol, 2006. 27(6): p. 652–7. [DOI] [PubMed] [Google Scholar]
- 29.Hashish N, et al. , Could 3D placental volume and perfusion indices measured at 11–14 weeks predict occurrence of preeclampsia in high-risk pregnant women? J Matern Fetal Neonatal Med, 2015. 28(9): p. 1094–8. [DOI] [PubMed] [Google Scholar]
- 30.Poon LC, et al. , Combined screening for preeclampsia and small for gestational age at 11–13 weeks. Fetal Diagn Ther, 2013. 33(1): p. 16–27. [DOI] [PubMed] [Google Scholar]
- 31.Hoogland HJ, de Haan J, and Martin CB, Placental size during early pregnancy and fetal outcome: a preliminary report of a sequential ultrasonographic study. Am J Obstet Gynecol, 1980. 138(4): p. 441–3. [DOI] [PubMed] [Google Scholar]
- 32.Wolf H, Oosting H, and Treffers PE, A longitudinal study of the relationship between placental and fetal growth as measured by ultrasonography. Am J Obstet Gynecol, 1989. 161(5): p. 1140–5. [DOI] [PubMed] [Google Scholar]
- 33.Azpurua H, et al. , Determination of placental weight using two-dimensional sonography and volumetric mathematic modeling. Am J Perinatol, 2010. 27(2): p. 151–5. [DOI] [PubMed] [Google Scholar]
- 34.Aye CY, et al. , Comparison of 2-D and 3-D estimates of placental volume in early pregnancy. Ultrasound Med Biol, 2015. 41(3): p. 734–40. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson GN, et al. , 3-D Ultrasound Segmentation of the Placenta Using the Random Walker Algorithm: Reliability and Agreement. Ultrasound Med Biol, 2015. 41(12): p. 3182–93. [DOI] [PubMed] [Google Scholar]
- 36.Cheong KB, et al. , Comparison of inter- and intraobserver agreement and reliability between three different types of placental volume measurement technique (XI VOCAL, VOCAL and multiplanar) and validity in the in-vitro setting. Ultrasound Obstet Gynecol, 2010. 36(2): p. 210–7. [DOI] [PubMed] [Google Scholar]
- 37.Grady L, Random walks for image segmentation. IEEE Trans Pattern Anal Mach Intell, 2006. 28(11): p. 1768–83. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson GN, et al. , A novel semi‐automated (SA) technique for 3D ultrasound measurement of placental volume. Ultrasound Obstet Gynecol, 2010(36): p. 82–82. [Google Scholar]
- 39.Looney P, et al. , <h1 aria-label=“vm.displayDocTitle” class=“document-title” style=“font-size: 1.6em; margin: 0px 10px 0px 0px; line-height: 1.3; color: rgb(51, 51, 51); font-family: sans-serif; outline: 0px !important;” tabindex=“0”> Automatic 3D ultrasound segmentation of the first trimester placenta using deep learning, in 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017). 2017: Melbourne; p. 279–282. [Google Scholar]
- 40.Looney P, et al. , Fully automated, real-time 3D ultrasound segmentation to estimate first trimester placental volume using deep learning. JCI insight, 2018. 11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Looney P, et al. , Prediction of term small for gestational age babies using first trimester placental volume; a comparison of a novel, fully automated technique, OxNNet with a commercially available, semi-automatic tool, VOCAL. 2018: Placenta (in print). [Google Scholar]
- 42.Collins SL, et al. , Rapid calculation of standardized placental volume at 11 to 13 weeks and the prediction of small for gestational age babies. Ultrasound Med Biol, 2013. 39(2): p. 253–60. [DOI] [PubMed] [Google Scholar]
- 43.Ipsen DC, Units, dimensions, and dimensionless numbers. 1960, New York ; London: McGraw-Hill. [Google Scholar]
- 44.Cohen-Overbeek T, Pearce JM, and Campbell S, The antenatal assessment of utero-placental and feto-placental blood flow using Doppler ultrasound. Ultrasound Med Biol, 1985. 11(2): p. 329–39. [DOI] [PubMed] [Google Scholar]
- 45.Brosens I, Dixon HG, and Robertson WB, Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol, 1977. 84(9): p. 656–63. [DOI] [PubMed] [Google Scholar]
- 46.Khong TY, et al. , Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol, 1986. 93(10): p. 1049–59. [DOI] [PubMed] [Google Scholar]
- 47.Khong Y and Brosens I, Defective deep placentation. Best Pract Res Clin Obstet Gynaecol, 2011. 25(3): p. 301–11. [DOI] [PubMed] [Google Scholar]
- 48.Schaaps JP, et al. , Shunting the intervillous space: new concepts in human uteroplacental vascularization. Am J Obstet Gynecol, 2005. 192(1): p. 323–32. [DOI] [PubMed] [Google Scholar]
- 49.Collins SL, et al. , Abdominal pregnancy: a perfusion confusion? Placenta, 2011. 32(10): p. 793–5. [DOI] [PubMed] [Google Scholar]
- 50.Clark AR, et al. , Understanding abnormal uterine artery Doppler waveforms: A novel computational model to explore potential causes within the utero-placental vasculature. Placenta, 2018. 66: p. 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matijevic R, et al. , Spiral artery blood flow in the central and peripheral areas of the placental bed in the second trimester. Obstet Gynecol, 1995. 86(2): p. 289–92. [DOI] [PubMed] [Google Scholar]
- 52.Ozkaya U, et al. , Doppler examination of uteroplacental circulation in early pregnancy: can it predict adverse outcome? J Clin Ultrasound, 2007. 35(7): p. 382–6. [DOI] [PubMed] [Google Scholar]
- 53.Kurjak A, et al. , Intervillous circulation in all three trimesters of normal pregnancy assessed by color Doppler. J Perinat Med, 1997. 25(4): p. 373–80. [DOI] [PubMed] [Google Scholar]
- 54.Jurkovic D, et al. , Transvaginal color Doppler assessment of the uteroplacental circulation in early pregnancy. Obstet Gynecol, 1991. 77(3): p. 365–9. [PubMed] [Google Scholar]
- 55.Hsieh YY, et al. , Longitudinal doppler sonographic measurements of vascular impedance in the central and peripheral spiral arteries throughout pregnancy. J Clin Ultrasound, 2000. 28(2): p. 78–82. [DOI] [PubMed] [Google Scholar]
- 56.Murakoshi T, et al. , Uterine and spiral artery flow velocity waveforms in pregnancy-induced hypertension and/or intrauterine growth retardation. Ultrasound Obstet Gynecol, 1996. 7(2): p. 122–8. [DOI] [PubMed] [Google Scholar]
- 57.Collins SL, Development of placental ultrasound markers to screen for the term, small for gestational age (SGA) baby, in Faculty of Medical Sciences. 2011, University of Oxford; p. 195. [Google Scholar]
- 58.Adler RS, On the relationship between power mode and pressure amplitude decorrelation. Ultrasound Med Biol, 2001. 27(9): p. 1291–6. [DOI] [PubMed] [Google Scholar]
- 59.Welsh A, Quantification of power Doppler and the index ‘fractional moving blood volume’ (FMBV). Ultrasound Obstet Gynecol, 2004. 23(4): p. 323–6. [DOI] [PubMed] [Google Scholar]
- 60.MacSweeney JE, Cosgrove DO, and Arenson J, Colour Doppler energy (power) mode ultrasound. Clin Radiol, 1996. 51(6): p. 387–90. [DOI] [PubMed] [Google Scholar]
- 61.Collins SL, et al. , Influence of power Doppler gain setting on Virtual Organ Computer-aided AnaLysis indices in vivo: can use of the individual sub-noise gain level optimize information? Ultrasound Obstet Gynecol, 2012. 40(1): p. 75–80. [DOI] [PubMed] [Google Scholar]
- 62.Hernandez‐Andrade E, et al. , Validation of fractional moving blood volume measurement with power Doppler ultrasound in an experimental sheep model. Ultrasound obstet gynecol, 2004. 23: p. 363–368. [DOI] [PubMed] [Google Scholar]
- 63.Welsh AW, et al. , Inapplicability of fractional moving blood volume technique to standardize Virtual Organ Computer-aided AnaLysis indices for quantified three-dimensional power Doppler. Ultrasound Obstet Gynecol, 2012. 40(6): p. 688–92. [DOI] [PubMed] [Google Scholar]
- 64.Stevenson GN, et al. , A technique for the estimation of fractional moving blood volume by using three-dimensional power Doppler US. Radiology, 2015. 274(1): p. 230–7. [DOI] [PubMed] [Google Scholar]
- 65.Collins S, et al. , 3D fractional moving blood volume (3D-FMBV) demonstrates decreased first trimester placental vascularity in pre-eclampsia but not the term, small for gestation age baby. PloS one, 2017. 12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]


