Ball et al. highlight the importance of Functional Cognitive Disorder for clinicians and researchers in the dementia field. They present a preliminary definition for this common condition, practical pointers to differential diagnosis, and highlight how identifying this condition can enhance studies of neurodegeneration.
Keywords: cognition, dementia, functional cognitive disorder, functional neurological disorder, mild cognitive impairment
Abstract
An increasing proportion of cognitive difficulties are recognized to have a functional cause, the chief clinical indicator of which is internal inconsistency. When these symptoms are impairing or distressing, and not better explained by other disorders, this can be conceptualized as a cognitive variant of functional neurological disorder, termed functional cognitive disorder (FCD). FCD is likely very common in clinical practice but may be under-diagnosed. Clinicians in many settings make liberal use of the descriptive term mild cognitive impairment (MCI) for those with cognitive difficulties not impairing enough to qualify as dementia. However, MCI is an aetiology-neutral description, which therefore includes patients with a wide range of underlying causes. Consequently, a proportion of MCI cases are due to non-neurodegenerative processes, including FCD. Indeed, significant numbers of patients diagnosed with MCI do not ‘convert’ to dementia. The lack of diagnostic specificity for MCI ‘non-progressors’ is a weakness inherent in framing MCI primarily within a deterministic neurodegenerative pathway. It is recognized that depression, anxiety and behavioural changes can represent a prodrome to neurodegeneration; empirical data are required to explore whether the same might hold for subsets of individuals with FCD. Clinicians and researchers can improve study efficacy and patient outcomes by viewing MCI as a descriptive term with a wide differential diagnosis, including potentially reversible components such as FCD. We present a preliminary definition of functional neurological disorder–cognitive subtype, explain its position in relation to other cognitive diagnoses and emerging biomarkers, highlight clinical features that can lead to positive diagnosis (as opposed to a diagnosis of exclusion), and red flags that should prompt consideration of alternative diagnoses. In the research setting, positive identifiers of FCD will enhance our recognition of individuals who are not in a neurodegenerative prodrome, while greater use of this diagnosis in clinical practice will facilitate personalized interventions.
Overlapping definitions
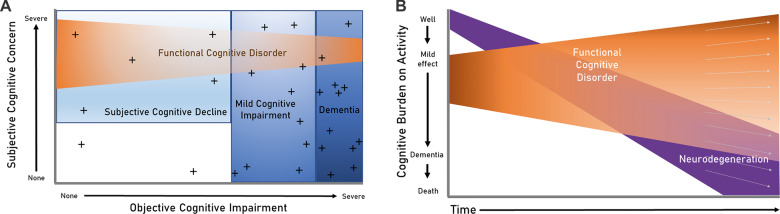
Functional cognitive disorder (FCD) refers to complaints of persistent problematic cognitive difficulties, when accompanied by positive features termed ‘internal inconsistency’ (Box 1), and which are not better explained by another disorder e.g. a neurodegenerative disease process (Box 2). This is relevant to all clinicians to whom such patients present, including in general practice, gerontology, neurology, psychiatry and others. FCD is likely common but is rarely diagnosed, perhaps in part because such patients usually concurrently meet descriptive criteria for either mild cognitive impairment (MCI), or subjective cognitive decline (SCD). MCI is a syndrome involving objective cognitive decline greater than expected for age that does not interfere with activities of daily life (Albert et al., 2011). SCD describes subjective concern regarding decline in cognitive abilities without evidence of objective cognitive deficit (Howard, 2020; Jessen et al., 2020). Conceptually, both SCD and MCI are heterogeneous concepts and include subjects with a variety of underlying causes (Blackburn et al., 2014), including neurodegenerative diseases, medical or psychiatric diagnoses, medication and alcohol or other recreational drug effects, and FCD (Fig. 1A). However, in practice, the majority of research involving MCI and/or SCD has been predicated on a linear progression from SCD through MCI to dementia, which is problematic if most of these patients do not in fact have underlying neurodegenerative disease.
Box 1.
Internal inconsistency
Internal inconsistency is the ability to perform a task well at certain times, but with significantly impaired ability at other times, particularly when the task is the focus of attention. Therefore, the individual components required to execute the task are intact, but there is difficulty engaging them at the appropriate intensity or duration on demand. We also considered whether a patient’s tendency to give ‘approximate answers’ should be used as an example of internal inconsistency. This may reflect differences in automatic versus explicit processing. This is not the same as simple fluctuation over time, which can be observed in many other processes (such as delirium, Lewy body disease, etc.). Finally, internal inconsistency needs to be demonstrated within a particular cognitive domain. Do not superficially take a cognitive screen summary score in the normal or mild range, plus a patient with significant day-to-day impairment, to conclude this is FCD (rather, this should be a starting point for exploring the particular cause of the day-to-day impairment).
Conversational abilities observed during interview (Alexander et al., 2019).
Reported activities, such as being involved in a cognitively demanding occupation; or difficulties only occurring in particular situations.
Collateral history suggesting concern is significantly higher in the individual than their supporter (including the ‘attended alone’ sign) (Bharambe and Larner, 2018b).
Specific patterns within neuropsychological testing that indicate cognitive processes performing better when accessed less explicitly, e.g. greater ability in delayed recall than initial registration of information.
Where examples such as the above are elicited, part of the diagnostic process should include pointing them out to the patient, and explaining that they demonstrate a temporary block to accessing memories, rather than a persistent memory defect.
Research is ongoing to investigate whether impaired meta-cognition (the ability to reflect on and monitor cognitive processes) may contribute to cognitive internal inconsistency (Bhome et al., 2019b).
We also considered whether a patient’s tendency to give ‘approximate answers’ should be used as an example of internal inconsistency. This tendency, the so-called Ganser syndrome, is poorly characterized in the literature, and care should be taken over what counts as an ‘approximate’ versus a ‘wrong’ answer. The key focus should be on a patient demonstrating normal and abnormal performance on the same cognitive ability, without there being other mitigating factors that intervene (e.g. fluctuations in consciousness, psychiatric state, or a significant headache).
Box 2.
Diagnostic criteria for functional neurological disorder: cognitive subtype
One or more symptoms of impaired cognitive function.
Clinical evidence of internal inconsistencya.
Symptoms or deficit that are not better explained by another medical or psychiatric disorderb.
Symptoms or deficit that cause clinically significant distress or impairmentc in social, occupational, or other important areas of functioning, or warrants medical evaluation.
a Box 1.
bPatients may have co-morbid medical or psychiatric disorders as well as FCD.
cTo aid reliability for neurodegenerative research purposes, a minimum of 6 months duration should be considered (refer to text).
Specify if: with/without a linked co-morbidity (refer to text).
Figure 1.
How FCD relates to other cognitive concepts. (A) Where FCD fits in relation to other key terminology used in the cognitive clinic. ‘Objective cognitive impairment’ denotes low scores on standardized testing. ‘Subjective cognitive concern’ denotes an individual’s perception of their cognitive difficulties (note some patients with MCI and dementia lack insight). Patients with FCD account for a proportion of those with MCI, and a proportion of those with SCD; rarely, those with FCD can meet criteria for dementia (i.e. severe enough to interfere with daily function and independence). Crosses represent biomarkers for neurodegenerative conditions. Biomarkers are clustered most densely among patients with dementia; a small number of true positive biomarkers also exist in the healthy population with neither subjective concerns nor objective impairment (indicating neurodegenerative tendency that has not yet manifested), and some will be false positives because a biomarker with 100% specificity seems unlikely (see McWhirter et al., 2020 for further discussion). (B) Trajectories in FCD (adapted from McWhirter et al., 2020). This illustrates the wide spectrum of potential trajectories within FCD, highlighting that some patients have considerable persisting symptoms and impairment even after serial testing, whereas others return to baseline functioning. The causes of these divergent trajectories may be explicable via co-morbidities or external factors, but often no such factors are identified. Disentangling this heterogeneity is an important area for future research. The x-axis represents each lifetime; those who remain above the x-axis to the end of their lifetime have died from other causes.
Biomarkers that predict Alzheimer’s pathology in particular, or neurodegeneration more generally (including but not limited to MRI and PET, genetics, and blood or CSF measurement of amyloid, tau and neurofilament) are already finding utility in clinical trials and are increasingly used in clinical practice. However, while biomarkers may provide evidence for or against a diagnosis of Alzheimer’s disease, a positive diagnosis of FCD on clinical grounds has a number of potentially important complementary roles. First, patients with FCD are likely to benefit from distinct strategies to help with their symptoms. Second, having FCD may prove to be an important exclusion criterion for clinical trials, or may need to be taken into account when interpreting the results of trials targeting Alzheimer’s pathology to reduce heterogeneity. Third, since a dual diagnosis of FCD and cognitive impairment secondary to Alzheimer’s pathology is entirely possible (indeed such dual diagnoses are common in other areas of neurology), optimal treatment strategies may need to focus both on FCD and Alzheimer’s pathology. And finally, as we move to diagnosing patients ever earlier, communicating biomarker results may precipitate FCD in individuals who would otherwise not have manifest symptoms for some time.
Patients with FCD are increasingly prevalent in tertiary memory clinics (comprising 12–56% of new referrals) (Elsey et al., 2015; Pennington et al., 2015a; Bharambe and Larner, 2018a; Wakefield et al., 2018; Bhome et al., 2019a; Pennington et al., 2019). Different case definitions may explain how some FCD case series score predominantly normally on objective cognitive testing, whereas others underperform or demonstrate inconsistencies in some areas of objective testing. Note that symptoms in FCD are not feigned. Where tested, patients with functional disorders do not consistently fail tests of performance validity or ‘effort’, but may display impaired selective attention (Teodoro et al., 2018). We encounter many patients who pass performance validity testing but score >2 standard deviations below normal on standardized cognitive testing (i.e. falling into the FCD/MCI overlap area on Fig. 1A). Population-based identification of MCI cases may over-recruit individuals with FCD, as they may be younger, more aware of research opportunities and more open to recruitment efforts.
De-emphasizing the inevitable expectation of progression to Alzheimer's dementia
Understanding the prodromal phase of dementia is clearly of great importance for elucidation of causal mechanisms and development of novel interventions for Alzheimer’s pathology. However, a substantial proportion of individuals with MCI will later return to normal cognitive function, or maintain stable cognition, rather than showing progressive deterioration. Neuropathological analyses of cohorts who met MCI criteria before death show they are intermediate between those with normal cognition and those with dementia (Stephan et al., 2012). In highlighting such associations, few reports focus on the substantial proportion of individuals with MCI whose brains are histologically normal (Schneider et al., 2009; Abner et al., 2017). It is also difficult to define a clear boundary between age-normative neuropathological changes and the burden of neurodegeneration that is required for cognitive impairment (Ferrer, 2012). There are many reasons why autopsy studies might miss very early neurodegeneration, such as subtle or not-yet-understood pathologies, varying degrees of immunohistochemical analysis and regional brain sampling (Nelson et al., 2012). Regardless, these factors do not fully explain the phenomenon of MCI in the presence of minimal or no brain pathology. In addition, many individuals with demonstrable neuropathological changes associated with Alzheimer’s disease identified after death did not experience cognitive symptoms in life (Latimer et al., 2017), raising the possibility that only a proportion of the cognitive symptoms experienced by those with neuropathology, might be caused by that pathology.
There is clearly a biological trajectory in Alzheimer’s disease, with the clinical syndrome usually preceded by an MCI phase (Jack et al., 2010). However, it is important not to extrapolate this backwards to assume that all or most subjects with MCI are on this trajectory en route to dementia, because this downplays the importance of other (including FCD) explanations for MCI. Many studies emphasize ‘conversion’ to dementia (e.g. annualized conversion rates of MCI to dementia), which implies a deterministic relationship between MCI and Alzheimer’s dementia (as well as implying an abrupt step-change). Biomarkers are increasingly being used to identify risk of clinical progression on an individual basis (van Maurik et al., 2019) but are, as yet, imperfect and not always available; and in general there tends to be less focus on the causes of cognitive symptoms in those who do not progress to dementia. A population-based analysis that tracked these changes over 7 years, found that 53% remained as MCI cases, while 35% reverted to normal cognition (Ganguli et al., 2019). A default assumption that neurodegeneration underlies MCI may be reinforced amongst clinicians and researchers who frequently interact with subject affected by established dementia (i.e. subjects who have passed through MCI as part of a neurodegenerative trajectory). In the wider population however, and especially in older subjects, other non-neurodegenerative aetiologies and multifactorial processes are likely to contribute significantly (Petersen et al., 2014). Figure 1B (adapted from McWhirter et al., 2020) illustrates how heterogeneous trajectories in FCD can account for some of the abovementioned discrepancy. Assumptions of progression may also contribute to widespread public anxiety regarding the inevitability of dementia.
Diagnosis and aetiology of functional cognitive disorder
Typical clinical presentations of FCD most commonly focus around memory impairment (often alongside attention and concentration difficulties), often in the form of ‘memory perfectionism’ and mnestic block (Pennington et al., 2015b). FCD less often involves non-amnestic cognitive functions such as praxis, language, or executive function. Current data suggest the typical age at onset of FCD is mid-life (therefore overlapping with early-onset neurodegeneration) (Pennington et al., 2015a; Bharambe and Larner, 2018a; Wakefield et al., 2018), but this may in part reflect the composition of specialist clinics, with referral patterns influenced by the increased likelihood of neurodegeneration in older ages. As with people in the prodromal stage of neurodegenerative dementia, those with FCD are often understandably anxious about their symptoms, are able to discuss their difficulties and coping strategies, and can display mild but persistent deficits (including those seen on objective standardized cognitive tests, or as observed by others in the general course of life), with few other clinical signs.
FCD definitions still lack consensus, hindering our understanding of prevalence particularly in community settings (Stone et al., 2015), and hindering wider understanding and acceptance of the diagnosis. Diagnostic difficulty around FCD exists for several reasons. First, the presence of mnestic concern, and the cognitive trajectory over the short term, may look similar across FCD and early neurodegeneration. Second, there is frequently co-occurrence of functional cognitive symptoms alongside some combination of neurodegeneration, general medical, psychiatric or surgical problems, or drug toxicity. In this context, the functional symptoms may be secondary, in the form of a ‘functional overlay’, although in the clinic setting it is often difficult to differentiate this from the background cognitive symptoms due to identified co-morbidities (including substances used). Unfortunately, this distinction is not aided by research studies that often exclude people with mental health conditions, despite their being very common in memory clinic. Third, FCD symptoms often persist over time (Schmidtke et al., 2008), so for example will still feature in MCI studies that check for the persistence of symptoms. Longer-term outcomes of FCD have not been thoroughly studied, although the default assumption should be that affected individuals have the same chance of later developing neurodegeneration as the background population (without such an occurrence indicating a ‘missed’ earlier diagnosis of neurodegeneration). However, this does require empirical testing, because in certain contexts FCD could arise as a prodrome to neurodegeneration (as has been found with certain presentations of late life anxiety, depression and mild behavioural impairment) (Livingston et al., 2017; Creese et al., 2019). These difficulties, and the recent entry of FCD into the cognitive diagnostic lexicon, likely explain why FCD is rarely diagnosed, despite its likely frequency, given the high prevalence of other functional neurological conditions (Carson and Lehn, 2016).
In addition to under-diagnosis due to diagnostic difficulty, some clinicians will be using other terms for the same condition in different settings (Blackburn et al., 2014; Bailey et al., 2017). Also, some clinicians may be avoiding naming the condition at all, or fall back on classifying the patient as either SCD or MCI (which are descriptive rather than aetiological categories). Some practitioners use the term ‘worried well’, presumably as a means of identifying a group of individuals whose symptoms are not due to underlying neurodegeneration. This is unsatisfactory to patients, who are generally not reassured when told their symptoms have no underlying pathological basis, but aren’t offered an alternative explanation. It also hinders efforts to positively identify a distinct group. The situation is improving with diagnostic systems e.g. Diagnostic and Statistical Manual, 5th edition (DSM-5) (American Psychiatric Association, 2013), recently switching to emphasize positive criteria for diagnosis rather than identifying functional neurological disorder (FND) solely by the absence of neurological, psychiatric or other general medical explanatory causes.
Here, we propose an operational definition for FCD (Box 2), which we hope will enable clearer communication in the clinical setting, and standardization for research purposes. This definition is in line with the DSM-5 definition of FND. The key to diagnosing FCD is identifying positive evidence of internal inconsistency (Box 1). However, we have also included a list of mimics (Box 3)—situations with a flavour of internal inconsistency but that should prompt consideration of alternative diagnoses. We recognize this is a changing field; these criteria represent a work in progress.
Box 3 Red flags to prompt consideration of diagnoses other than functional cognitive disorder (and why)
FCD is common and most clinicians who interact with patients with cognitive difficulties should be confident at identifying it. It is important not to medicalize normal human experience, for example where cognitive concerns are found in the absence of objective deficit, and where this is not associated with distress nor impairment. The following are some features that should prompt consideration of certain differential diagnoses.
Greater difficulty understanding single words than the superficially more complex task of whole sentence comprehension (this is a feature of semantic dementia).
Difficulties pertaining primarily to visual comprehension [posterior cortical atrophy can produce difficulties that mimic internal inconsistency, including the reverse size phenomenon, and perception of moving versus static objects (Crutch et al., 2012)].
Apathy or low mood can also cause discrepancy between real-world behaviour and reported deficits (for example in depression or frontal meningioma). For example, in response to ‘Where did you go on holiday' receiving a sparse response such as ‘Provence' without the patient being able to move from this to spontaneously generate more specific information; yet he can, on direct questioning, recall specific events once these are mentioned by his wife.
Intact implicit memory with defective conscious memory, can occur in conditions such as Korsakoff’s psychosis.
Difficulties greater on recognition than on recall, may be a consequence of damage to perirhinal or parahippocampal areas (Eichenbaum et al., 2007).
Difficulty in real-world executive functioning out of proportion to superficial pencil-and-paper testing, can be a feature of dorsolateral prefrontal damage.
Long term temporal pattern: Absence of decline, or fluctuation over months or years. Such a pattern indicates incongruity with neurodegeneration, but by itself is not a positive identifier for FCD, since other processes could cause this.
Variability day-to-day should lead to consideration of conditions such as obstructive sleep apnoea, delirium or Lewy body disease (if other appropriate features are present). Typically patients with these conditions would not display normal and abnormal performance on similar tasks within a single consultation.
Sudden onset and persistence should lead to consideration of stroke syndromes. Semantic access dyslexia is a left-hemisphere stroke syndrome that typically causes inconsistency in identifying the same semantic stimulus presented multiple times (this is distinct from semantic dementia, in which the semantic concepts are consistently non-retrievable) (Mirman and Britt, 2014).
Finally, have a higher suspicion for neurodegeneration if the presentation is non-mnestic, particularly since early-onset Alzheimer’s disease has relatively more non-mnestic presentations (Koedam et al., 2010).
It is important to note that DSM-5 FND includes only sensory and motor (not cognitive) phenotypes. We envisage FCD as the equivalent cognitive phenotype (and we would recommend DSM to consider this in their next revision). Placing FCD within the broader FND umbrella recognizes the phenotypic overlap across functional disorders, which includes similarities in neurocognitive profiles (Teodoro et al., 2018). Thus the ‘cognitive fog’ often described by patients with functional movement disorder or dissociative seizures can be conceptualized as part of the same broad condition. Although our mechanistic understanding of FND is incomplete, it is notable that neurobiological models of FND make no distinction between the mechanism of different symptom types. Motor, sensory, cognitive and interoceptive symptoms can all conceivably arise from the same basic malfunction proposed to occur in FND, which is entirely consistent with the common co-occurrence of multiple functional symptoms in the same individual (Edwards et al., 2012; Van den Bergh et al., 2017).
We also feel DSM’s ‘associated features supporting diagnosis’ for FND generally apply to FCD in particular, namely: a history of multiple somatic symptoms; stress or trauma at onset; and dissociative symptoms (though none of these features are necessary for diagnosis, and absence should not lead to the diagnosis being withheld). Finally, we also feel it is helpful to include a specifier for presence or absence of any co-morbidity that is linked to the cognitive symptoms. A non-exhaustive list includes health anxiety, mild traumatic brain injury (mTBI), depression, fibromyalgia or Alzheimer’s pathology. Such co-morbidities can influence the way people with FCD present, and the types of interventions they might respond to. As an illustration, systematic reviews have suggested that whilst mTBI is sometimes accompanied by temporary effects on attention, processing speed and memory, there is evidence of good recovery beyond the initial weeks and months (Carroll et al., 2014; Cassidy et al., 2014). This makes it possible that many of the self-reported symptoms outside this time frame may have a functional disorder aetiology. The situation is often clarified by the clinician’s reassessment of the reported severity of the head injury and surrounding circumstances; a cognitive behavioural therapy framework is often helpful to understand how expectations may drive behavioural responses to the injury (van Gils et al., 2020). An operational definition of FCD provides the opportunity for the TBI field to quantify the prevalence of a functional component to cognitive symptomatology.
In cognitive clinics, patients with FCD are typically encountered following symptom duration of at least 6 months. However, there is no clear need to wait for this duration before making an FCD diagnosis if positive indicators are present. Recent-onset cases may be harder to diagnose than persistent cases, and this would alter the differential diagnosis. It would also be important to avoid over-diagnosis of short-lived forgetting that is within the normal human experience. However, substantial clinical benefit could be gained from making and communicating an FCD diagnosis early, rather than subjecting the patient to prolonged diagnostic limbo.
Substantial heterogeneity in severity can be seen within FCD, as illustrated in Fig. 1A and B. Depending on the level of associated impairment, FCD cases may often additionally meet the definition of one of SCD, MCI or dementia. However, these purely descriptive classifications should be used with great caution (regardless of suspected underlying aetiology). This is because they have come to be associated with progressive neuropathology; if, however, the cognitive presentation is being driven by a functional disorder, then greater impairment does not have the same implications regarding irreversible progression. The adoption of a definition for FCD opens the door to testing whether an ‘FCD subtype of MCI’ would contribute to sample stratification in biomarker or intervention studies, and also aid communication of likely outcome and potential treatment.
A diagnosis of FCD would be excluded if another condition better accounted for the symptoms, such as cognitive symptoms that occur as part of a depressive episode, sometimes termed ‘depressive pseudo-dementia’. The temporal relationship, severity of depression, and the pattern of impairments can inform this distinction. Note that cognitive symptoms may not resolve on depressive episode resolution (Rock et al., 2014). Of patients referred to a tertiary neuropsychiatry clinic, half of those meeting FCD criteria had co-morbid depression (and therefore half did not) (Bhome et al., 2019a). In addition, subthreshold generalized anxiety disorder, dysthymia, and obsessive-compulsive personality traits are commonly noted and appear to be aetiologically relevant in many cases. We hope that our definition can enable research to better quantify rates and relevance of co-morbidities and other external factors, in FCD and in comparison to those in other groups (such as healthy controls, and those with early neurodegeneration). Patients with functional disorders often find themselves falling between different specialties, and individual clinicians often feel they are not best placed to offer management. We consider that clinicians working in all specialties that diagnose cognitive disorders should have the skills to recognize FCD, and can play an important part in its management (Carson et al., 2016). Heterogeneity within FCD means that some patients may be relatively straightforward to identify, and management should begin with an explanation of the symptoms and giving a positive diagnosis; others may require referral tailored to unravelling a diagnostic challenge; and others may be best managed within a mental health model.
We also considered whether FCD could fit within DSM-5’s somatic symptom disorder (SSD). However, SSD does not actually capture elements of FCD that we feel are integral (i.e. internal inconsistency), so does nothing to aetiologically disentangle FCD from prodromal Alzheimer’s disease (which can involve similar levels of anxiety). SSD also does not account for those with FCD without a significant anxiety component.
Better appreciation of functional cognitive disorder would enhance outcomes across the cognitive field
Research is ongoing to identify positive features in clinical assessment that point to a functional cognitive diagnosis (for a review see McWhirter et al., 2020). When found, it is usually helpful to transparently discuss these internal inconsistencies and their implications with the patient (Stone and Edwards, 2012). These features can also be used to form testable hypotheses. For example, we could predict that among individuals with cognitive symptoms, those displaying internal inconsistency would be: (i) more likely to respond to certain treatments (e.g. treatments to modify metacognition); (ii) more likely to remain stable or improve their cognitive scores, and less likely to eventually develop dementia; and (iii) less likely to have biomarkers of Alzheimer’s or global neurodegeneration.
It may actually be easier to identify those who meet criteria for FCD, than those who have underlying Alzheimer’s pathology, due to the limited access and imperfect precision of current Alzheimer’s biomarkers. In other words, neurodegeneration clinical trial candidates should not just meet SCD or MCI criteria, but also lack the positive features of functional cognitive conditions, in order to enhance power to detect effective Alzheimer’s disease modifiers. On the other hand, to understand processes and efficacy at the population level, particularly in the older age bracket, it may be more appropriate to use dimensional scales (rather than exclusions) to quantify the separate effects of co-morbidities, drug toxicity, psychological and lifestyle factors, and FCD.
Improving our identification of key characteristics of FCD, and the many often interwoven aetiologies behind MCI, should simultaneously improve identification of those who are in the prodromal stage of neurodegeneration. Doing so requires thorough assessment of other likely aetiological contributors, as well as examining patterns of ‘reversion’ as well as ‘conversion’. This could provide greater signal relative to noise, both in understanding biological processes of neurodegeneration, and in testing interventions. Establishing FCD as an essential axis in cognitive assessment will help us to better understand, and ultimately modify, the causes of cognitive impairment, and to determine who will and who will not develop dementia.
Funding
H.A.B. is funded by a National Institute for Health Research (NIHR) Clinical Lectureship. R.B. is supported by a NIHR Academic Clinical Fellowship. D.J.B. is funded by Alzheimer's Research UK & Rosetrees. D.J.B., M.R. and A.V. acknowledge the support of the National Institute for Health Research Sheffield Biomedical Research Centre (Translational Neuroscience). M.J.E. is funded by NIHR and the Medical Research Council. S.M.F. is funded by the Wellcome Trust & the Royal Society. J.H. is funded by a Wellcome Clinical Research Career Development Fellowship. R.H., N.C.F., J.M.S. and M.N.R. acknowledge the support of the National Institute for Health Research University College London Hospitals Biomedical Research Centre. L.M. receives philanthropic funding from Baillie Gifford. T.R.N. is funded by an NIHR Clinician Scientist Award. C.P. is funded by the Innovative Medicines Initiative, BRACE, and the David Telling Charitable Trust. J.S. is supported by a National Research Scotland Career Researcher Fellowship. This publication presents independent research; the views expressed are those of the authors and not necessarily those of the specified funding bodies.
Competing interests
A.J.C. runs a not for profit website www.headinjurysymptoms.org, is a paid associate editor of JNNP, is unpaid treasurer of the functional neurological disorders society, and gives independent testimony in court on a range of topics including functional cognitive disorders. J.D.I. received an honorarium for an advisory board for Biogen on treatments for Alzheimer's disease, has received conference expenses from Roche and has been Principal Investigator on clinical trials in Alzheimer's disease funded by Roche, Merck and Lupin Pharmaceuticals. L.M. provides independent medical testimony in court cases regarding patients with functional disorders. M.R. has received speaker’s fees from UCB Pharma, Eisai and LivaNova, and benefitted from an educational grant from UCB Pharma; he receives payments from Elsevier as Editor-in-Chief of Seizure, and authorship fees for book publications from Oxford University Press. J.S. reports independent expert testimony work for personal injury and medical negligence claims, royalties from UpToDate for articles on functional neurological disorder, is unpaid secretary of the Functional Neurological Disorder Society and runs a free non-profit self-help website for FND, www.neurosymptoms.org. A.V. has received consulting fees and travel support from Biogen and Merck.
Glossary
- FCD =
functional cognitive disorder;
- FND =
functional neurological disorder;
- MCI =
mild cognitive impairment;
- SCD =
subjective cognitive decline
References
- Abner EL, Kryscio RJ, Schmitt FA, Fardo DW, Moga DC, Ighodaro ET, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol 2017; 81: 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M, Blackburn D, Reuber M.. Patients' accounts of memory lapses in interactions between neurologists and patients with functional memory disorders. Sociol Health Illn 2019; 41: 249–65. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Somatic Symptom and Related Disorders. Diagnostic and statistical manual of mental disorders (DSM-5). 5th edn Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- Bailey C, Bell SM, Blackburn DM.. How the UK describes functional memory symptoms. Psychogeriatrics 2017; 17: 336–7. [DOI] [PubMed] [Google Scholar]
- Bharambe V, Larner AJ.. Functional cognitive disorders: demographic and clinical features contribute to a positive diagnosis. Neurodegener Dis Manag 2018. a; 8: 377–83. [DOI] [PubMed] [Google Scholar]
- Bharambe V, Larner AJ.. Functional cognitive disorders: memory clinic study. Prog Neurol Psychiatry 2018. b; 22: 19–22. [Google Scholar]
- Bhome R, Huntley JD, Price G, Howard RJ.. Clinical presentation and neuropsychological profiles of Functional Cognitive Disorder patients with and without co-morbid depression. Cogn Neuropsychiatry 2019. a; 24: 152–64. [DOI] [PubMed] [Google Scholar]
- Bhome R, Mcwilliams A, Huntley JD, Fleming SM, Howard RJ. Metacognition in functional cognitive disorder- a potential mechanism and treatment target. Cogn Neuropsychiatry 2019. b; 24: 311–21. [DOI] [PubMed] [Google Scholar]
- Bhome R, McWilliams A, Huntley JD, Fleming SM, Howard RJ.. Metacognition in functional cognitive disorder- a potential mechanism and treatment target. Cogn Neuropsychiatry 2019. b; 24: 311–21. [DOI] [PubMed] [Google Scholar]
- Blackburn DJ, Wakefield S, Shanks MF, Harkness K, Reuber M, Venneri A.. Memory difficulties are not always a sign of incipient dementia: a review of the possible causes of loss of memory efficiency. Br Med Bull 2014; 112: 71–81. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Cancelliere C, Cote P, Hincapie CA, Kristman VL, et al. Systematic review of the prognosis after mild traumatic brain injury in adults: cognitive, psychiatric, and mortality outcomes: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014; 95 (3Suppl): S152–73. [DOI] [PubMed] [Google Scholar]
- Carson A, Hallett M, Stone J.. Assessment of patients with functional neurologic disorders. Handb Clin Neurol 2016; 139: 169–88. [DOI] [PubMed] [Google Scholar]
- Carson A, Lehn A.. Epidemiology Handb Clin Neurol 2016; 139: 47–60. [DOI] [PubMed] [Google Scholar]
- Cassidy JD, Cancelliere C, Carroll LJ, Cote P, Hincapie CA, Holm LW, et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014; 95: Suppl): S132–51. [DOI] [PubMed] [Google Scholar]
- Creese B, Brooker H, Ismail Z, Wesnes KA, Hampshire A, Khan Z, et al. Mild Behavioral Impairment as a Marker of Cognitive Decline in Cognitively Normal Older Adults. The American journal of geriatric psychiatry: official journal of the American Association for. Geriatric Psychiatry 2019; 27: 823–34. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC.. Posterior cortical atrophy. Lancet Neurol 2012; 11: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ.. A Bayesian account of ‘hysteria’. Brain 2012; 135: 3495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C.. The medial temporal lobe and recognition memory. Annu Rev Neurosci 2007; 30: 123–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsey C, Drew P, Jones D, Blackburn D, Wakefield S, Harkness K, et al. Towards diagnostic conversational profiles of patients presenting with dementia or functional memory disorders to memory clinics. Patient Educ Couns 2015; 98: 1071–7. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog Neurobiol 2012; 97: 38–51. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Jia Y, Hughes TF, Snitz BE, Chang C-C, Berman SB, et al. Mild cognitive impairment that does not progress to dementia: a population-based study. J Am Geriatr Soc 2019; 67: 232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. Subjective cognitive decline: what is it good for? Lancet Neurol 2020; 19: 203–4. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010; 9: 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, et al. The characterisation of subjective cognitive decline. Lancet Neurol 2020; 19: 271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA.. Early versus late-onset Alzheimer's disease: more than age alone. J Alzheimers Dis 2010; 19: 1401–8. [DOI] [PubMed] [Google Scholar]
- Latimer CS, Keene CD, Flanagan ME, Hemmy LS, Lim KO, White LR, et al. Resistance to Alzheimer disease neuropathologic changes and apparent cognitive resilience in the nun and Honolulu-Asia aging studies. J Neuropathol Exp Neurol 2017; 76: 458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017; 390: 2673–734. [DOI] [PubMed] [Google Scholar]
- McWhirter L, Ritchie C, Stone J, Carson A.. Functional cognitive disorders: a systematic review. Lancet Psychiatry2020; 7: P191–207. [DOI] [PubMed] [Google Scholar]
- Mirman D, Britt AE.. What we talk about when we talk about access deficits. Phil Trans R Soc B 2014; 369: 20120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012; 71: 362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington C, Ball H, Swirski M.. Functional cognitive disorder: diagnostic challenges and future directions. Diagnostics (Basel) 2019; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington C, Hayre A, Newson M, Coulthard E.. Functional cognitive disorder: a common cause of subjective cognitive symptoms. J Alzheimers Dis 2015. a; 48: S19–S24. [DOI] [PubMed] [Google Scholar]
- Pennington C, Newson M, Hayre A, Coulthard E.. Functional cognitive disorder: what is it and what to do about it? Pract Neurol 2015. b; 15: 436–44. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L.. Mild cognitive impairment: a concept in evolution. J Intern Med 2014; 275: 214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD.. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 2014; 44: 2029–40. [DOI] [PubMed] [Google Scholar]
- Schmidtke K, Pohlmann S, Metternich B.. The syndrome of functional memory disorder: definition, etiology, and natural course. Am J Geriatr Psychiatry 2008; 16: 981–8. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA.. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009; 66: 200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan BC, Hunter S, Harris D, Llewellyn DJ, Siervo M, Matthews FE, et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry 2012; 17: 1056–76. [DOI] [PubMed] [Google Scholar]
- Stone J, Edwards M.. Trick or treat? Showing patients with functional (psychogenic) motor symptoms their physical signs. Neurology 2012; 79: 282–4. [DOI] [PubMed] [Google Scholar]
- Stone J, Pal S, Blackburn D, Reuber M, Thekkumpurath P, Carson A.. Functional (Psychogenic) cognitive disorders: a perspective from the neurology clinic. J Alzheimers Dis 2015; 48: S5–S17. [DOI] [PubMed] [Google Scholar]
- Teodoro T, Edwards MJ, Isaacs JD.. A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: systematic review. J Neurol Neurosurg Psychiatry 2018; 89: 1308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh O, Witthöft M, Petersen S, Brown RJ.. Symptoms and the body: taking the inferential leap. Neurosci Biobehav Rev 2017; 74: 185–203. [DOI] [PubMed] [Google Scholar]
- van Gils A, Stone J, Welch K, Davidson LR, Kerslake D, Caesar D, et al. Management of mild traumatic brain injury. Pract Neurol 2020; 1–9. doi: practneurol-2018-002087. [DOI] [PubMed] [Google Scholar]
- van Maurik IS, Vos SJ, Bos I, Bouwman FH, Teunissen CE, Scheltens P, et al. Biomarker-based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurol 2019; 18: 1034–44. [DOI] [PubMed] [Google Scholar]
- Wakefield SJ, Blackburn DJ, Harkness K, Khan A, Reuber M, Venneri A.. Distinctive neuropsychological profiles differentiate patients with functional memory disorder from patients with amnestic-mild cognitive impairment. Acta Neuropsychiatr 2018; 30: 90–6. [DOI] [PubMed] [Google Scholar]