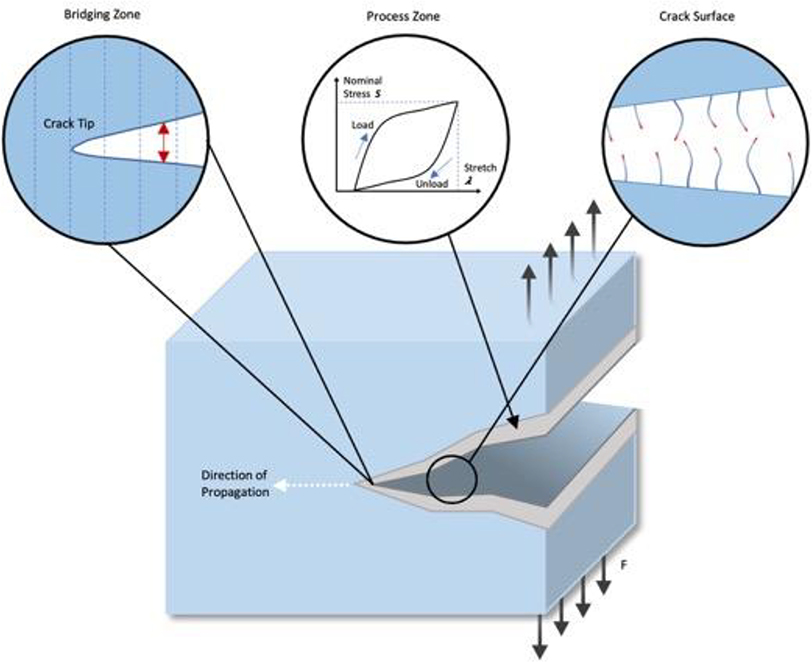
Figure 2. Schematic representation of crack propagation in a hydrogel network.
When a crack forms, energy is released and transferred to the crack tip. The crack will continue to propagate if this energy is sufficient to rupture the polymer chains lying across the crack plane. The fracture energy of hydrogels is divided into two parts: fracture energy from polymer chains rupturing at the crack surface, and the mechanical energy dissipated by loading/unloading the hydrogel in the process zone. Adapted with permission.[26] Copyright 2013, The Royal Chemistry Society.