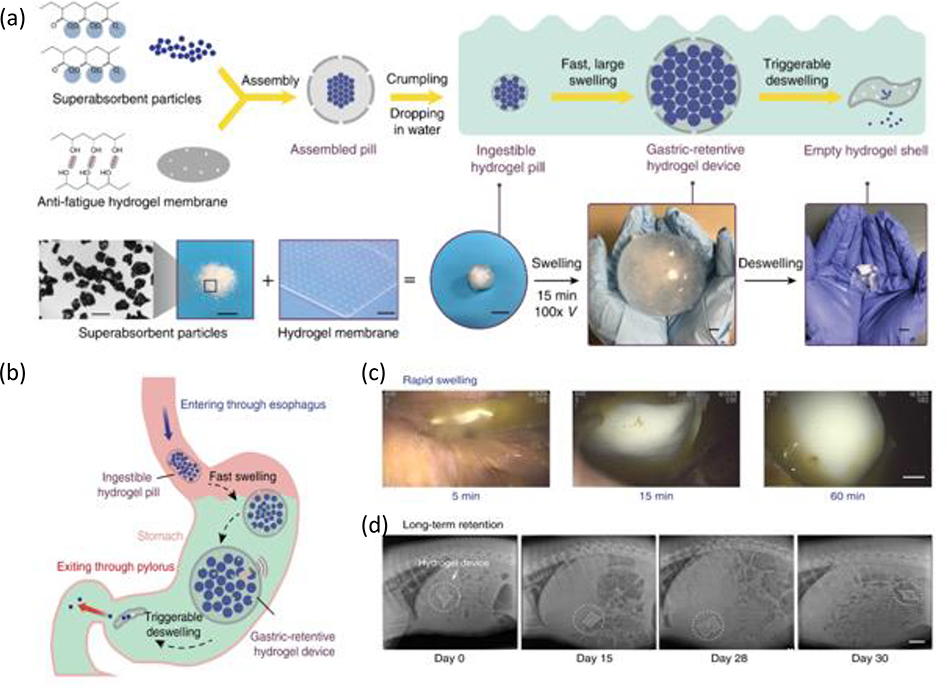
Figure 8. Ingestible hydrogel device for long term gastric retention and physiological monitoring.
a) Schematic and images of the synthesis and functionalities of the hydrogel device. b) Proposed mechanism of action of the gastric-retentive hydrogel device as it enters the stomach through the esophagus as hydrogel pill, swells and is retained within the stomach for a prolonged period, and finally exits through the pylorus in the form of a shrunken capsule and small particles. c) Endoscopic images demonstrating rapid swelling of the hydrogel device as it resides within the porcine stomach. d) X-ray images demonstrating the residence of the hydrogel device within the porcine stomach before eventually being emptied into the GI tract (shown here for a period of 29 days within the stomach). Adapted with permission.[137] Copyright 2019, Springer Nature.