Abstract
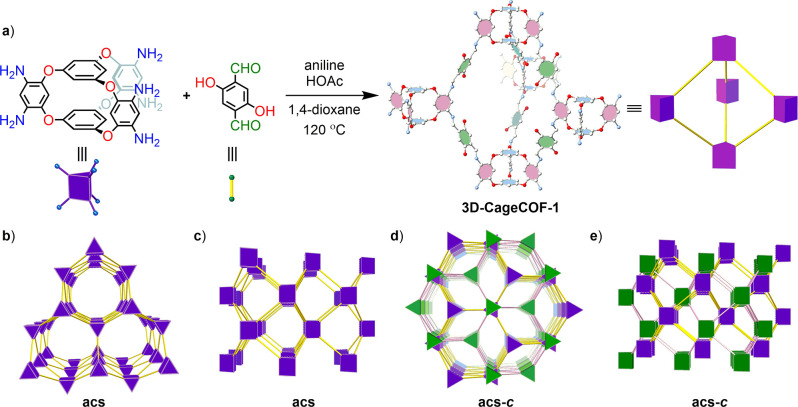
Three-dimensional (3D) covalent organic frameworks (COFs) are rare because there is a limited choice of organic building blocks that offer multiple reactive sites in a polyhedral geometry. Here, we synthesized an organic cage molecule (Cage-6-NH2) that was used as a triangular prism node to yield the first cage-based 3D COF, 3D-CageCOF-1. This COF adopts an unreported 2-fold interpenetrated acs topology and exhibits reversible dynamic behavior, switching between a small-pore (sp) structure and a large-pore (lp) structure. It also shows high CO2 uptake and captures water at low humidity (<40%). This demonstrates the potential for expanding the structural complexity of 3D COFs by using organic cages as the building units.
Introduction
Covalent organic frameworks (COFs) have emerged as a versatile class of crystalline porous solids that can be rationally designed and fine-tuned by synthesis to enhance their functionality.1−4 The framework topology of a COF is predetermined by the connectivity and geometry of the building blocks, and, as for metal–organic frameworks (MOFs),5,6 COF synthesis is often tolerant to changes in linker size and chemical functionality. As such, different COF topologies (or nets) can be purposefully targeted by a judicious choice of building blocks.
To date, the majority of reported COFs are two-dimensional (2D) layered structures with 1D pore channels, where strong noncovalent interactions modulate the interlayer packings.7 By contrast, COFs with 3D nets require more than one nonplanar building unit, and these have proven to be less synthetically accessible.4,8 Consequently, only eight distinct 3D nets in the Reticular Chemistry Structure Resource (RCSR)9 have been reported for COFs: dia,10lon,11bor,2ctn,2pts,12rra,13srs,14 and ffc.15 The first six of these were constructed using tetrahedral building blocks with 4 points of extension, such as tetraphenylmethane, tetraphenylsilane, adamantane, and their derivatives.4 This is in stark contrast to endeavors that have realized topologically more complex MOF nets,16 where metal clusters with up to 24 points of extension have been reported.17,18 However, organic molecules rarely have a high number (>4) of reactive sites that are spatially arranged to allow for extended interconnection. This is a major reason for the difficulty in broadening the range of topologies for COFs, and it is a central challenge for expanding the functional scope of porous 3D frameworks.
As with other porous 3D solids, such as zeolites,19 MOFs,6,20 and porous organic polymers,21 the interconnected pore channels in 3D COFs may provide opportunities for superior function,22,23 such as enhanced molecular diffusivity. New functions may also be possible: for example, inserting rotatable dipolar linkers into certain framework topologies could afford COFs with a net response to electric fields.24 Moreover, many porous 3D framework structures have been shown to exhibit structural flexibility in response to adsorption, as exemplified by breathing MOFs25 and swellable porous polymers.26 Such complementary and cooperative phenomena in porous solids can be beneficial in applications such as controlled drug delivery, gas capture, and size-selective catalysis.27−29 A key step to unlocking new applications for COF materials is to increase the chemical and structural diversity—for example, by accessing new framework topologies using polyhedral organic nodes.
Organic cage molecules can be viewed as polyhedrons.30 Moreover, the vertexes of a cage polyhedron may be functionalized to link up with another building block for framework structures31 or to expand its connectivity (e.g., 12-arm octahedral cages32). Cages are therefore exciting candidates for constructing 3D COFs with highly connected topologies. However, this strategy remains largely unexplored because it is challenging to design and synthesize organic cage molecules that are compatible with reticular chemistry, shape-persistent, and stable under COF synthetic conditions, not least because organic cage molecules are often also assembled using dynamic covalent chemistry.33 The first organic-cage-based COF was only recently reported, and this was a 2D net.34 The cage molecule was used as a triangular node to connect with a linear linker, yielding the prototypical 2D hcb net. To the best of our knowledge, the use of organic cage molecules with higher connectivity (>3 connecting sites) to generate 3D COFs has not yet been reported.
Here, we synthesized a shape-persistent organic cage with six pendant amine groups positioned in a trigonal prismatic arrangement (Cage-6-NH2, Figure 1). By reacting Cage-6-NH2 with the linear 2,5-dihydroxyterephthalaldehyde (DHTPA), a 3D COF, 3D-CageCOF-1, adopting the acs topology was synthesized. In the extended structure of 3D-CageCOF-1, there are two interpenetrated acs nets. 3D-CageCOF-1 was found to undergo reversible transformations between a small-pore (sp) structure and a large-pore (lp) structure in response to guest loading of dimethylformamide (DMF). Also, this hydrophilic 3D-CageCOF-1 exhibited a high water uptake (22 wt %) at low relative humidity (40%) and a high carbon dioxide uptake at 1 bar (204 mg/g, 273 K).
Figure 1.
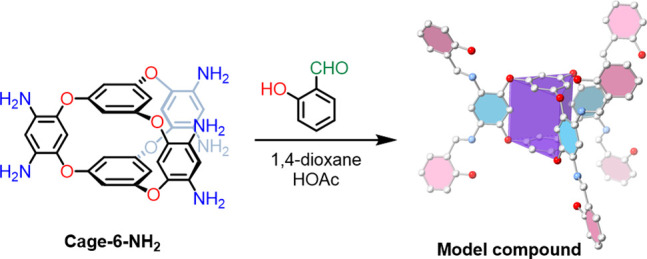
Synthesis of model compound and its single-crystal structure. Single-crystal atom colors: C, gray; N, blue; O, red. H atoms are omitted for clarity.
Results and Discussion
The discovery of triangular prism-shaped building blocks was important for increasing the topological diversity of MOFs.35−38 However, triangular prisms have not been used in COF synthesis because of the limited availability of suitable building blocks. To address this, we synthesized the triangular prism-shaped Cage-6-NH2 in two steps from a previously reported hexanitro precursor that was reported to have D3h symmetry (Supporting Information, Schemes S1 and S2).39 Importantly, the cage structure of Cage-6-NH2 does not contain imine or boronate ester bonds, which means that Cage-6-NH2 is compatible, in principle, with a wide range of synthetic conditions for COF formation. In addition, unlike planar molecular building blocks that usually lead to layered COFs, Cage-6-NH2 is decorated with 6 amine groups in a 3D trigonal prismatic arrangement that are ready to be extended in three dimensions.
The 3D symmetry of the amine groups in Cage-6-NH2 was initially investigated in a solution using 1H and 13C NMR spectroscopy (Figures S1–S4), which indicated that the cage had D3h symmetry like the previously reported hexanitro precursor.39 To investigate this further, we synthesized a model compound by reacting Cage-6-NH2 with 2-hydroxybenzaldehyde, which has a phenol group that is capable of forming hydrogen bonds with the cage molecule (Figure 1). NMR spectra of the model compound (Figures S6) again indicated that the model compound had D3h symmetry in solution, and the triangular prism shape of the cage was confirmed by single-crystal X-ray structure (Figure 1 and Table S5 for refinement details). In the single-crystal structure of the model compound, the pendant amine groups were distorted away from a perfect trigonal prismatic geometry, although their geometry is likely to have been affected by the close packing of the pendant aromatic groups in the crystal structure (Figure S42).
The trigonal prismatic geometry of Cage-6-NH2, along with its shape persistence in the solid state, makes it a promising candidate for constructing several 3D topologies, such as stp, tpt, sit, acs, and nia.40 Here we used the combination of Cage-6-NH2 with a linear linker to target COFs with acs nets according to reticular chemistry.35 DHTPA was chosen as the linear linker on the basis that intramolecular hydrogen bonds between the hydroxyl groups of DHTPA and the imine bonds in the COF product might be beneficial for directing the supramolecular assembly of the building blocks during synthesis and for stabilizing the resulting COF structure.41 Atomistic COF models were constructed using Cage-6-NH2 and DHTPA as building blocks and the acs net as the blueprint (Figure 2).42 Models of both the noninterpenetrated framework structure and its 2-fold and 3-fold interpenetrated forms were built (Figure S26). In contrast with tetrahedral-based building units, which tend to form COFs with high degrees of interpenetrations,11,43 the bulky core of Cage-6-NH2 enables the maximum interpenetration to be limited to 3-fold, thus simplifying the task of solving the structure by simulation, assuming that an acs net is formed. The underlying structure of 3D-CageCOF-1 was analyzed by powder X-ray diffraction (PXRD) combined with the structural models, produced using the principles of reticular chemistry.
Figure 2.
(a) Scheme for the synthesis of 3D-CageCOF-1 from Cage-6-NH2 and DHTPA, which can be topologically represented as a triangular prism and a linear strut, respectively. Model atom colors: C, white; N, blue; O, red. H atoms are omitted for clarity. (b, c) Two views of an acs crystal net, where the purple nodes represent the cage-based building blocks; (d, e) two comparable views of the 2-fold interpenetrated acs-c net (c, catenated), with the cage nodes belonging to the different nets colored in purple or green.
Despite screening several hydrothermal reaction conditions for the reaction between Cage-6-NH2 and DHTPA (Figures S7 and S8), we initially only isolated poorly crystalline samples that had weak PXRD patterns with a single peak at 5.3° (Cu-Kα; see Figures S7 and S8). The nitrogen sorption-derived Brunauer–Emmett–Teller (BET) surface areas for these poorly crystalline samples at 77 K were also low (ca. 230 m2 g–1; Figures S12 and S13). We believe that the poor crystallinity of the product was due to the poor solubilities of Cage-6-NH2 and the oligomeric byproducts in the reaction solvent, which hindered reversibility and network self-healing during the COF synthesis. Inspired by dynamic covalent chemistry-derived approaches for COF synthesis, aniline was employed as a modulator.11 After optimizing the synthetic conditions by including 7.5 mol. equiv. of aniline per Cage-6-NH2 as a modulator and using 6 M HOAc as the catalyst, we produced a COF, 3D-CageCOF-1, with much improved crystallinity (Figures S9–S11).
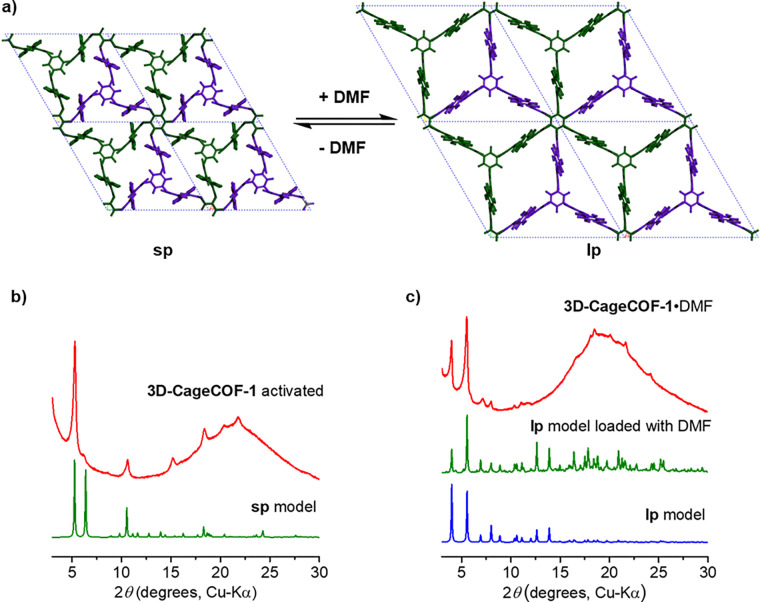
The crystallinity of activated 3D-CageCOF-1 was characterized by PXRD. By treating the reflections at 2.82° and 5.65° (λ = 0.825015 Å) as the [100] and [200] reflections, respectively, in a trigonal/hexagonal symmetry structure, we were able to determine that the likely a = b cell edges of the activated 3D-CageCOF-1 structure were ∼19.4 Å (Figure S27), in agreement with the symmetry and cell dimensions that were calculated for the activated model with P3̅ symmetry. Unfortunately, it was not possible to unambiguously determine the c unit cell edge from our PXRD data and, hence, to produce a meaningful fit, despite performing this measurement using synchrotron radiation (Figure S27). However, by using our structural models we were able to propose a 2-fold interpenetrated acs-c net as the most likely structure for 3D-CageCOF-1. The noninterpenetrated structure was optimized to the a = b cell length of 24.0 Å, which is much larger than the experimental values. Catenation of two such COF networks led to a significant reduction in the cell volume, owing to the strong noncovalent interactions between the two networks, thus resulting in a contracted structure referred to as a small-pore (sp) model in Figure 3 and hereafter. The 3-fold interpenetration also resulted in a contracted cell, similar in size to the 2-fold cell. A comparison of these simulated models with the experiment indicated that 3D-CageCOF-1 adopted an sp, 2-fold interpenetrated structure (Figure 3a and Figure S29).
Figure 3.
(a) Scheme showing the reversible structural transformation between the small-pore (sp) and large-pore (lp) forms of 3D-CageCOF-1 upon loading and the removal of DMF solvent. (b) Comparison of the experimental PXRD pattern of activated 3D-CageCOF-1 with the simulated pattern for the sp, 2-fold model. (c) Comparison of the experimental PXRD pattern collected on the DMF solvate of 3D-CageCOF-1 with two simulated patterns. The empty lp structure was optimized with the cell parameters fixed at the experimental values; the optimized lp structure was then loaded with DMF up to saturation at room temperature and 1 bar pressure.
Nitrogen sorption analysis at 77 K revealed that 3D-CageCOF-1 is microporous and has a type-II sorption isotherm (Figure S14) with a pore volume of 0.50 cm3 g–1. The BET and Langmuir surface areas calculated for 3D-CageCOF-1 were 1040 and 1336 m2 g–1, respectively (Figures S15 and S16). The measured BET surface area equates to 54% of the theoretical calculated nitrogen-accessible surface area for the sp, 2-fold model shown in Figure 3b. The pore diameters derived by fitting to the nitrogen isotherm are 8.8 and 5.6 Å, in close agreement with the pore diameters of the proposed model (8.5 and 5.1 Å; Figure S17 and Table S3 for comparison with other models). Furthermore, the simulated nitrogen adsorption isotherm uptake at 1 bar for the sp, 2-fold structure is also in very good agreement with the experimental counterpart (Figure S31).
Scanning electronic microscopy (SEM) indicated that activated 3D-CageCOF-1 has a flakelike morphology (Figure S32). Solid-state NMR cross-polarization magic-angle spinning (CP/MAS) confirmed the formation of imine bonds at 164 ppm (Figure S35). Thermogravimetric analysis (TGA) (Figure S36a) and differential scanning calorimetry (DSC) (Figure S36b) indicated that the COF starts to decompose at 400 °C.
Dynamic behaviors are common in 3D network materials, and this is particularly well studied and documented for MOFs.44,45 Previous studies have also revealed that 3D COF materials can reversibly respond to stimuli, giving rise to applications in sensing46 and adsorption.27,47,48 Initially, to investigate the chemical stability of 3D-CageCOF-1, we immersed powdered samples in a series of common organic solvents (MeOH, CH3CN, tetrahydrofuran (THF), and DMF) and water for 24 h, washed the samples by Soxhlet with acetone, and then dried the samples in air. On the basis of PXRD patterns and Fourier transform infrared (FT-IR) data recorded after these experiments, we found that the structure and crystallinity of 3D-CageCOF-1 remained intact after being reactivated from these solvents (Figures S38 and S39). However, on inspection of the solvated PXRD patterns, we found that the powdered COF sample immersed in DMF solvent transformed into a new phase, 3D-CageCOF-1·DMF, which we characterized by recording PXRD patterns on solvated samples, suspended in DMF solvent, loaded in glass capillaries (Figure 3c).
To calculate a structural model for 3D-CageCOF-1·DMF, we initially used the DMF solvated PXRD pattern to determine potential unit cell dimensions. A close similarity was observed between the P3̅ model and the experimental PXRD data that refined to the unit cell dimensions: a = b = 25.5 Å, c = 22.9 Å, and V = 12 880 Å3 (see Figure S28 for Pawley refinement (Rp = 0.8%, Rwp = 1.4%, and χ2 = 2.6%)). On the basis of the experimental cell, a large-pore (lp), 2-fold structure was constructed and optimized, adopting the same P3̅ symmetry. This lp model transformed into the sp model for the activated phase without fixing the unit cell parameters at the experimental values (Figure 3b; see Video 1 in the Supporting Information, which shows the lp model transforming into the sp model). Simulated PXRD patterns, using the empty or DMF-loaded lp structure, are in good agreement with the pattern of 3D-CageCOF-1·DMF (Figure 3c).
3D-CageCOF-1·DMF transformed back into the activated sp structure after the DMF solvent molecules were thermally removed from the COF via in situ heating at 175 °C (Figure 40). Our structural modeling suggests that the reversible transformations between the activated sp and DMF-solvated lp phases are a result of the structure being stabilized by either the interframework interactions, leading to corrugated frameworks, or the presence of DMF solvent, allowing the frameworks to stay expanded. This flexible behavior is facilitated by a combination of the shape persistence of the cage core, the hingelike motions of the cage “arms” (oxygen-bridged phenyls), and the rotation of the imine bonds in the COF networks.
Activated 3D-CageCOF-1 has a relatively small pore size and the pores are highly decorated with oxygen and nitrogen atoms, in principle providing a favorable polar, hydrophilic environment for guests such as CO2 and H2O. To support this, 3D-CageCOF-1 has a maximum CO2 uptake of 204 mg g–1 at 273 K and 1 bar, and 107 mg g–1 at 298 K and 1 bar (Figure 4a), which are among the highest values reported for COFs under these conditions (Figure S20).49−513D-CageCOF-1 also exhibits an S-shaped water vapor sorption isotherm with a small desorption hysteresis at 298 K, which is a desirable sorption behavior for water capture by nanoporous solids.52 In addition, 3D-CageCOF-1 shows a steep pore filling in the pressure (P/P0) range of 0.21–0.34, achieving an adsorption capacity of 22 wt % at P/P0 = 0.4 and a maximum capacity of 33 wt % (Figure 4b); this is comparable with the best performing COF material (COF-432) reported for water harvesting.52 Also, there was no marked loss in performance after three adsorption–desorption cycles (Figure S23), suggesting that 3D-CageCOF-1, or analogues thereof, could be a promising candidate for water capture.
Figure 4.

(a) CO2 isotherms and (b) water vapor sorption isotherms for 3D-CageCOF-1.
Conclusion
In conclusion, we have synthesized a 3D organic-cage-based COF for the first time, 3D-CageCOF-1, which adopts the acs crystal net in a 2-fold interpenetrated form. Our strategy leverages a rationally designed organic cage molecule that is shape-persistent and offers six reactive sites, arranged in a triangular prism geometry, that are ready for extension in three dimensions. 3D-CageCOF-1 exhibits dynamic behavior toward DMF and shows promising performance for water harvesting and CO2 uptake. This work affords a new strategy for expanding the library of COF building units and enriching the range of network topologies that can be accessed. It also demonstrates the potential for embedding organic cage molecules into COFs to discover new functional materials, for example, by designing cages that can impart additional functionality, such as preformed pores to bind specific guests.
Acknowledgments
For funding, the authors acknowledge the Engineering and Physical Sciences Research Council (EPSRC) (EP/N004884/1) and the Leverhulme Trust via the Leverhulme Research Centre for Functional Materials Design. The authors acknowledge Diamond Light Source for access to beamlines I19 (CY21726) and I11 (CY23666). We thank Dr. A. Ken Inge and Mr. Erik Svensson Grape at the University of Stockholm for attempting to collect electron diffraction data for 3D-CageCOF-1. We thank Dr. Michael E. Briggs and Prof. Jeffrey L. Katz for useful discussions related to the synthesis of Cage-6-NH2. We thank Prof. Rochus Schmid for providing the weaver code; Mr. Xiaowei Zhu for help preparing the figures; and Dr. Luca Catalona for help performing DSC.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c07732.
Computational methodology, powder and single-crystal X-ray diffraction, SEM, TEM, DSC, TGA, and gas and vapor sorption analysis (PDF)
X-ray crystallographic data of Cage-6-NO2 (CIF)
X-ray crystallographic data of model compound (CIF)
lp model transforming into the sp model (MP4)
Computation models for sp and lp structures (ZIP)
The authors declare no competing financial interest.
Supplementary Material
References
- Cote A. P.; Benin A. I.; Ockwig N. W.; O’Keeffe M.; Matzger A. J.; Yaghi O. M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. 10.1126/science.1120411. [DOI] [PubMed] [Google Scholar]
- El-Kaderi H. M.; Hunt J. R.; Mendoza-Cortes J. L.; Cote A. P.; Taylor R. E.; O’Keeffe M.; Yaghi O. M. Designed Synthesis of 3D Covalent Organic Frameworks. Science 2007, 316, 268–272. 10.1126/science.1139915. [DOI] [PubMed] [Google Scholar]
- Geng K.; He T.; Liu R.; Dalapati S.; Tan K. T.; Li Z.; Tao S.; Gong Y.; Jiang Q.; Jiang D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814. 10.1021/acs.chemrev.9b00550. [DOI] [PubMed] [Google Scholar]
- Guan X.; Chen F.; Fang Q.; Qiu S. Design and Applications of Three Dimensional Covalent Organic Frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. 10.1039/C9CS00911F. [DOI] [PubMed] [Google Scholar]
- Yaghi O. M.; O’Keeffe M.; Ockwig N. W.; Chae H. K.; Eddaoudi M.; Kim J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Ma X.; Scott T. F. Approaches and Challenges in the Synthesis of Three-Dimensional Covalent-Organic Frameworks. Commun. Chem. 2018, 1, 98. 10.1038/s42004-018-0098-8. [DOI] [Google Scholar]
- Beuerle F.; Gole B. Covalent Organic Frameworks and Cage Compounds: Design and Applications of Polymeric and Discrete Organic Scaffolds. Angew. Chem., Int. Ed. 2018, 57, 4850–4878. 10.1002/anie.201710190. [DOI] [PubMed] [Google Scholar]
- O’Keeffe M.; Peskov M. A.; Ramsden S. J.; Yaghi O. M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. 10.1021/ar800124u. [DOI] [PubMed] [Google Scholar]
- Uribe-Romo F. J.; Hunt J. R.; Furukawa H.; Klock C.; O’Keeffe M.; Yaghi O. M. A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. 10.1021/ja8096256. [DOI] [PubMed] [Google Scholar]
- Ma T.; Kapustin E. A.; Yin S. X.; Liang L.; Zhou Z.; Niu J.; Li L.-H.; Wang Y.; Su J.; Li J.; Wang X.; Wang W. D.; Wang W.; Sun J.; Yaghi O. M. Single-Crystal X-ray Diffraction Structures of Covalent Organic Frameworks. Science 2018, 361, 48–52. 10.1126/science.aat7679. [DOI] [PubMed] [Google Scholar]
- Lin G.; Ding H.; Yuan D.; Wang B.; Wang C. A Pyrene-Based, Fluorescent Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138, 3302–3305. 10.1021/jacs.6b00652. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Duan J.; Ma D.; Li P.; Li S.; Li H.; Zhou J.; Ma X.; Feng X.; Wang B. Three-Dimensional Anionic Cyclodextrin-Based Covalent Organic Frameworks. Angew. Chem., Int. Ed. 2017, 56, 16313–16317. 10.1002/anie.201710633. [DOI] [PubMed] [Google Scholar]
- Yahiaoui O.; Fitch A. N.; Hoffmann F.; Froba M.; Thomas A.; Roeser J. 3D Anionic Silicate Covalent Organic Framework with srs Topology. J. Am. Chem. Soc. 2018, 140, 5330–5333. 10.1021/jacs.8b01774. [DOI] [PubMed] [Google Scholar]
- Lan Y.; Han X.; Tong M.; Huang H.; Yang Q.; Liu D.; Zhao X.; Zhong C. Materials Genomics Methods for High-Throughput Construction of COFs and Targeted Synthesis. Nat. Commun. 2018, 9, 5274. 10.1038/s41467-018-07720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Jiang H.; Li M.; O’Keeffe M.; Eddaoudi M. Reticular Chemistry 3.2: Typical Minimal Edge-Transitive Derived and Related Nets for the Design and Synthesis of Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8039. 10.1021/acs.chemrev.9b00648. [DOI] [PubMed] [Google Scholar]
- Du D. Y.; Qin J. S.; Sun Z.; Yan L. K.; O’Keeffe M.; Su Z. M.; Li S. L.; Wang X. H.; Wang X. L.; Lan Y. Q. An Unprecedented (3,4,24)-Connected Heteropolyoxozincate Organic Framework as Heterogeneous Crystalline Lewis Acid Catalyst for Biodiesel Production. Sci. Rep. 2013, 3, 2616. 10.1038/srep02616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillerm V.; Kim D.; Eubank J. F.; Luebke R.; Liu X.; Adil K.; Lah M. S.; Eddaoudi M. A Supermolecular Building Approachfor the Design and Construction of Metal–Organic Frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. 10.1039/C4CS00135D. [DOI] [PubMed] [Google Scholar]
- Wright P. A.Microporous Framework Solids; Royal Society of Chemistry: Cambridge, 2008. [Google Scholar]
- Li M.; Li D.; O’Keeffe M.; Yaghi O. M. Topological Analysis of Metal-Organic Frameworks with Polytopic Linkers and/or Multiple Building Units and the Minimal Transitivity Principle. Chem. Rev. 2014, 114, 1343–1370. 10.1021/cr400392k. [DOI] [PubMed] [Google Scholar]
- Lee J. M.; Cooper A. I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120, 2171–2214. 10.1021/acs.chemrev.9b00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Lin R. B.; Wang J.; Wang B.; Liang B.; Yildirim T.; Zhang J.; Zhou W.; Chen B. Optimization of the Pore Structures of MOFs for Record High Hydrogen Volumetric Working Capacity. Adv. Mater. 2020, 32, 1907995. 10.1002/adma.201907995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Chang J.; Li S.; Guan X.; Li D.; Li C.; Tang L.; Xue M.; Yan Y.; Valtchev V.; Qiu S.; Fang Q. Three-Dimensional Tetrathiafulvalene-Based Covalent Organic Frameworks for Tunable Electrical Conductivity. J. Am. Chem. Soc. 2019, 141, 13324–13329. 10.1021/jacs.9b06908. [DOI] [PubMed] [Google Scholar]
- Durholt J. P.; Jahromi B. F.; Schmid R. Tuning the Electric Field Response of MOFs by Rotatable Dipolar Linkers. ACS Cent. Sci. 2019, 5, 1440–1448. 10.1021/acscentsci.9b00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B. Q.; Yang Q. Y.; Wang S. Q.; Vandichel M.; Kumar A.; Crowley C.; Kumar N.; Deng C. H.; GasconPerez V.; Lusi M.; Wu H.; Zhou W.; Zaworotko M. J. Reversible Switching between Nonporous and Porous Phases of a New SIFSIX Coordination Network Induced by a Flexible Linker Ligand. J. Am. Chem. Soc. 2020, 142, 6896–6901. 10.1021/jacs.0c01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Shi Z. L.; Wei L.; Zhou B.; Tan J.; Zhou H. L.; Zhang Y. B. Guest-Dependent Dynamics in a 3D Covalent Organic Framework. J. Am. Chem. Soc. 2019, 141, 3298–3303. 10.1021/jacs.8b13691. [DOI] [PubMed] [Google Scholar]
- Ma Y. X.; Li Z. J.; Wei L.; Ding S. Y.; Zhang Y. B.; Wang W. A Dynamic Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2017, 139, 4995–4998. 10.1021/jacs.7b01097. [DOI] [PubMed] [Google Scholar]
- Horike S.; Shimomura S.; Kitagawa S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. 10.1038/nchem.444. [DOI] [PubMed] [Google Scholar]
- Horcajada P.; Serre C.; Maurin G.; Ramsahye N. A.; Balas F.; Vallet-Regi M.; Sebban M.; Taulelle F.; Férey G. Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. 10.1021/ja710973k. [DOI] [PubMed] [Google Scholar]
- Santolini V.; Miklitz M.; Berardo E.; Jelfs K. E. Topological Landscapes of Porous Organic Cages. Nanoscale 2017, 9, 5280–5298. 10.1039/C7NR00703E. [DOI] [PubMed] [Google Scholar]
- Swamy S. I.; Bacsa J.; Jones J. T. A.; Stylianou K. C.; Steiner A.; Ritchie L. K.; Hasell T.; Gould J. A.; Laybourn A.; Khimyak Y. Z.; Adams D. J.; Rosseinsky M. J.; Cooper A. I. A Metal-Organic Framework with a Covalently Prefabricated Porous Organic Linker. J. Am. Chem. Soc. 2010, 132, 12773–12775. 10.1021/ja104083y. [DOI] [PubMed] [Google Scholar]
- Culshaw J. L.; Cheng G.; Schmidtmann M.; Hasell T.; Liu M.; Adams D. J.; Cooper A. I. Dodecaamide Cages: Organic 12-arm Building Blocks for Supramolecular Chemistry. J. Am. Chem. Soc. 2013, 135, 10007–10010. 10.1021/ja403987j. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Mastalerz M. Organic Cage Compounds-From Shape-Persistency to Function. Chem. Soc. Rev. 2014, 43, 1934–1947. 10.1039/C3CS60358J. [DOI] [PubMed] [Google Scholar]
- Ma J. X.; Li J.; Chen Y. F.; Ning R.; Ao Y. F.; Liu J. M.; Sun J.; Wang D. X.; Wang Q. Q. Cage Based Crystalline Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 3843–3848. 10.1021/jacs.9b00665. [DOI] [PubMed] [Google Scholar]
- Sudik A. C.; Cote A. P.; Yaghi O. M. Metal-Organic Frameworks Based on Trigonal Prismatic Building Blocks and the New ″acs″ Topology. Inorg. Chem. 2005, 44, 2998–3000. 10.1021/ic050064g. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Li P.; Anderson R.; Wang X.; Zhang X.; Robison L.; Redfern L. R.; Moribe S.; Islamoglu T.; Gómez-Gualdrón D. A.; Yildirim T.; Stoddart J. F.; Farha O. K. Balancing Volumetric and Gravimetric Uptake in Highly Porous Materials for Clean Energy. Science 2020, 368, 297–303. 10.1126/science.aaz8881. [DOI] [PubMed] [Google Scholar]
- Schoedel A.; Zaworotko M. J. [M3(μ3-O)(O2CR)6] and Related Trigonal Prisms: Versatile Molecular Building Blocks for Crystal Engineering of Metal–Organic Material Platforms. Chem. Sci. 2014, 5, 1269–1282. 10.1039/C4SC00171K. [DOI] [Google Scholar]
- Chen Z.; Li P.; Zhang X.; Li P.; Wasson M. C.; Islamoglu T.; Stoddart J. F.; Farha O. K. Reticular Access to Highly Porous acs-MOFs with Rigid Trigonal Prismatic Linkers for Water Sorption. J. Am. Chem. Soc. 2019, 141, 2900–2905. 10.1021/jacs.8b13710. [DOI] [PubMed] [Google Scholar]
- Katz J. L.; Selby K. J.; Conry R. R. A Single-Step Synthesis of D3h-Symmetric Bicyclooxacalixarenes. Org. Lett. 2005, 7, 3505–3507. 10.1021/ol051180q. [DOI] [PubMed] [Google Scholar]
- Kalmutzki M. J.; Hanikel N.; Yaghi O. M. Secondary Building Units as the Turning Point in the Development of the Reticular Chemistry of MOFs. Sci. Adv. 2018, 4, eaat9180. 10.1126/sciadv.aat9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandambeth S.; Shinde D. B.; Panda M. K.; Lukose B.; Heine T.; Banerjee R. Enhancement of Chemical Stability and Crystallinity in Porphyrin-Containing Covalent Organic Frameworks by Intramolecular Hydrogen Bonds. Angew. Chem., Int. Ed. 2013, 52, 13052–10356. 10.1002/anie.201306775. [DOI] [PubMed] [Google Scholar]
- Keupp J.; Schmid R. TopoFF: MOF Structure Prediction Using Specifically Optimized Blueprints. Faraday Discuss. 2018, 211, 79–101. 10.1039/C8FD00051D. [DOI] [PubMed] [Google Scholar]
- Guan X.; Ma Y.; Li H.; Yusran Y.; Xue M.; Fang Q.; Yan Y.; Valtchev V.; Qiu S. Fast, Ambient Temperature and Pressure Ionothermal Synthesis of Three-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 4494–4498. 10.1021/jacs.8b01320. [DOI] [PubMed] [Google Scholar]
- Lee J. H.; Jeoung S.; Chung Y. G.; Moon H. R. Elucidation of Flexible Metal-Organic Frameworks: Research Progresses and Recent Developments. Coord. Chem. Rev. 2019, 389, 161–188. 10.1016/j.ccr.2019.03.008. [DOI] [Google Scholar]
- Peh S. B.; Karmakar A.; Zhao D. Multiscale Design of Flexible Metal–Organic Frameworks. Trends in Chemistry 2020, 2, 199–213. 10.1016/j.trechm.2019.10.007. [DOI] [Google Scholar]
- Yanai N.; Kitayama K.; Hijikata Y.; Sato H.; Matsuda R.; Kubota Y.; Takata M.; Mizuno M.; Uemura T.; Kitagawa S. Gas detection by structural variations of fluorescent guest molecules in a flexible porous coordination polymer. Nat. Mater. 2011, 10, 787–793. 10.1038/nmat3104. [DOI] [PubMed] [Google Scholar]
- Wang H.; Li B.; Wu H.; Hu T. L.; Yao Z.; Zhou W.; Xiang S.; Chen B. A Flexible Microporous Hydrogen-Bonded Organic Framework for Gas Sorption and Separation. J. Am. Chem. Soc. 2015, 137, 9963–9970. 10.1021/jacs.5b05644. [DOI] [PubMed] [Google Scholar]
- Choi H. S.; Suh M. P. Highly Selective CO2 Capture in Flexible 3D Coordination Polymer Networks. Angew. Chem., Int. Ed. 2009, 48, 6865–6869. 10.1002/anie.200902836. [DOI] [PubMed] [Google Scholar]
- Ozdemir J.; Mosleh I.; Abolhassani M.; Greenlee L. F.; Beitle R. R.; Beyzavi M. H. Covalent Organic Frameworks for the Capture, Fixation, or Reduction of CO2. Front. Energy Res. 2019, 7, 77. 10.3389/fenrg.2019.00077. [DOI] [Google Scholar]
- Li H.; Pan Q.; Ma Y.; Guan X.; Xue M.; Fang Q.; Yan Y.; Valtchev V.; Qiu S. Three-Dimensional Covalent Organic Frameworks with Dual Linkages for Bifunctional Cascade Catalysis. J. Am. Chem. Soc. 2016, 138, 14783–14788. 10.1021/jacs.6b09563. [DOI] [PubMed] [Google Scholar]
- Gao Q.; Li X.; Ning G.-H.; Xu H.-S.; Liu C.; Tian B.; Tang W.; Loh K. P. Covalent Organic Framework with Frustrated Bonding Network for Enhanced Carbon Dioxide Storage. Chem. Mater. 2018, 30, 1762–1768. 10.1021/acs.chemmater.8b00117. [DOI] [Google Scholar]
- Nguyen H. L.; Hanikel N.; Lyle S. J.; Zhu C.; Proserpio D. M.; Yaghi O. M. A Porous Covalent Organic Framework with Voided Square Grid Topology for Atmospheric Water Harvesting. J. Am. Chem. Soc. 2020, 142, 2218–2221. 10.1021/jacs.9b13094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.