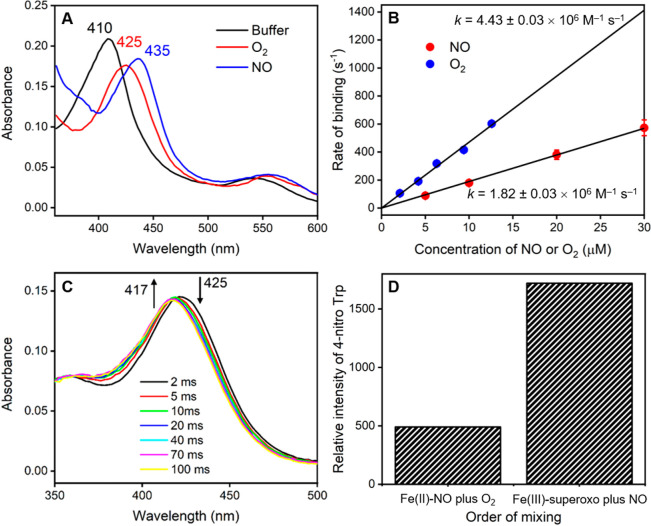
Figure 2.
Stopped-flow analysis of the mechanism of gas binding in TxtE. (a) Absorbance spectra measured after 50 ms using stopped-flow photodiode array measurements upon mixing of TxtE iron(II) with either anaerobic buffer, 20 μM O2, or 20 μM NO. (b) Dependence of the rate constant for gas binding to iron(II) heme TxtE on the concentration of either O2 or NO. The data were fitted to a straight line to determine the 2nd order rate constant for O2 or NO binding. (c) Time-resolved spectra at selected time points from double mixing stopped-flow experiments, where iron(II) heme TxtE was first mixed with 20 μM O2, aged for 50 ms, and then mixed with 20 μM NO prior to data collection. The iron(III)-superoxo species at 425 nm is rapidly converted to the water-ligated iron(III) form at 417 nm over 100 ms. (d) Relative amounts of the l-4-nitrotryptophan product formed in samples collected from double mixing stopped-flow experiments, where iron(II) heme TxtE was first mixed with either 20 μM O2 or 20 μM NO, aged for 50 ms, and then mixed with 100 μM NO or O2, respectively. All raw data can be found in the Supporting Information.