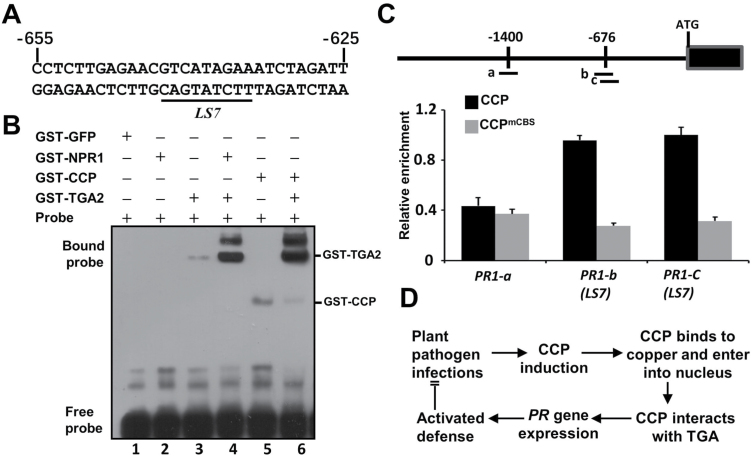
Fig. 6.
CCP enhances the binding activity of TGA2 to the LS7 element of the PR1 promoter in vivo. (A) The nucleotide sequence of the LS7 element (underlined) and surrounding region in the PR1 promoter. The numbers on the sequence indicate the position of the nucleotide relative to the transcription start site of the PR1 mRNA. The sequence was used as a probe in EMSAs. (B) EMSA showing the binding of TGA2 with the LS7 element of the PR1 promoter. GST-tagged GFP, TGA2, NPR1, and CCP proteins were purified from E. coli and mixed in different combinations. Equal amounts of the biotin-labeled LS7 element were incubated with different protein combinations, and the DNA binding was analyzed by EMSAs. (C) ChIP-qPCR assays showing binding of CCP to fragments of the PR1 promoter in vivo. Chromatins from 35S:CCP and 35S:CCPmCBS transgenic Arabidopsis seedlings with anti-Flag beads were collected for DNA extraction and quantitative PCR assays. Non-transgenic Col-0 plants acted as a negative control. Mean ±SD, n=3. Top, diagram of the PR1 promoter and the fragments for Chip-qPCR. (D) A proposed working model of CCP-mediated defense activation. Upon plant pathogen challenges, CCP is induced and binds to copper. CCP is translocated into the nucleus and interacts with TGA2, resulting in induction of defense response genes and improved immunity against plant pathogens.