FIGURE 2.
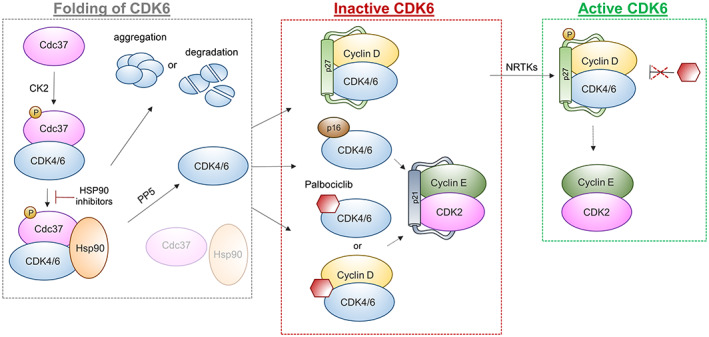
Regulation of CDK4 and CDK6. Phosphorylation of co‐chaperone cell division cycle 37 (Cdc37) by the casein kinase 2 (CK2) enables binding to CDK4/6 and complex formation with heat shock protein 90 (Hsp90), which facilitates accurate protein folding. Inhibition of HSP90 leads to aggregation or degradation of unfolded kinases. Dephosphorylation of Cdc37 by phosphatase protein phosphatase 5 (PP5) releases CDK4/6 and allows complex formation with different regulatory subunits. CDK4/6, Cyclin D and Cip/Kip proteins (p27) form an inactive trimeric complex, which gets activated upon phosphorylation of p27 by nonreceptor tyrosine kinases (NRTKs) and translocates into the nucleus. Association with inhibitors, such as p16 or palbociclib, drives the formation of inactive complexes and indirectly promotes inactivation of CDK2
