FIGURE 2.
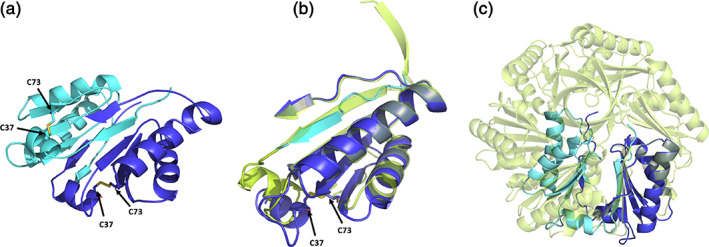
A comparison of a novel CutR dimeric form to the traditional flat hexameric form of the same protein. (a) Cartoon representation of the observed novel dimeric form. A disulfide bridge forms between C37 and C73, pulling the N‐terminus from its traditional position in the BMC domain, causing residues 18–28 to occupy the adjacent monomer by domain swapping. (b) Overlay of a monomer from the hexameric form (lime green) with residues 18–28 of one monomer (cyan) and residues 29–116 from the second monomer (deep blue), with emphasis on regions that differ. (c) Superimposition of one chain from the dimer with one chain from the flat hexamer shows that severe steric clashes would be caused by the presence of the dimeric form in the context of the hexameric assembly
