FIGURE 3.
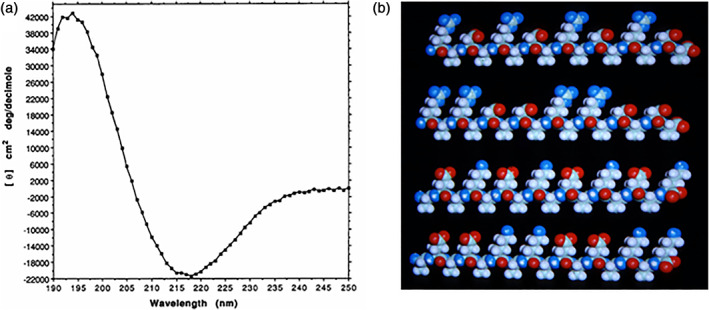
The first self‐assembling peptide. (a) CD spectrum of the first self‐assembling EAK16 peptide (AcN‐AEAEAKAKAEAEAKAK‐CONH2). A typical β‐sheet CD spectrum with a minimum at 218 nm and a maximum at 195 nm is observed. (b) and the molecular models of the designer amphiphilic self‐assembling peptides. These peptides have two distinctive sides, one hydrophobic and the other hydrophilic. The hydrophobic side forms a double sheet inside of the fiber and the hydrophilic side forms the outside of the nanofibers that interacts with water molecules, forming hydrogel that contains as high as 99.9% water. At least three types of molecules can be made, with −, +, −/+ on the hydrophilic side. The individual self‐assembling peptide molecules are ~6 nm long. The first such peptide, EAK16‐II, was discovered from a yeast protein, Zuotin. 5 , 9 This peptide inspired us to systematically design a large class of self‐assembling peptides. When these peptides are dissolved in water in the presence of salt, they spontaneously assemble into well‐ordered nanofibers and then further into nanofiber scaffold hydrogels
