FIGURE 7.
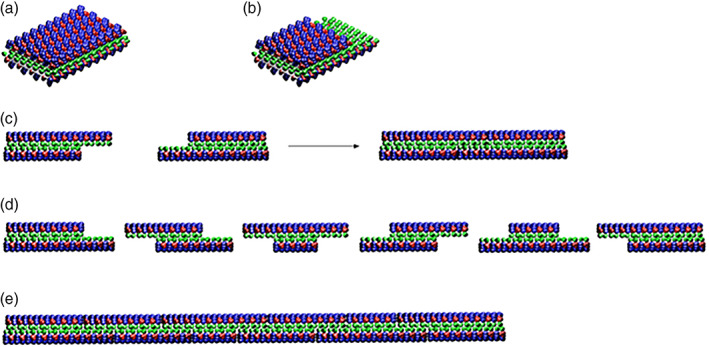
Molecular model for dynamic reassembly. When the peptides self‐assemble into stable β‐sheets in water, they form intermolecular hydrogen bonds along the peptide backbones. The β‐sheet structure has two distinctive sides, one hydrophobic with an array of alanines (green color) and the other with amino acids of negatively charged (red color) and positively charged (blue color). The alanines form overlap packed hydrophobic interactions in water, a structure that is found in silk fibroin from silkworm and spiders. On the charged sides, both positive and negative charges are packed together through intermolecular ionic interactions in a checkerboard‐like manner. When the fragments of nanofiber first meet, the hydrophobic sides may not fit perfectly but with gaps. However, the non‐specific hydrophobic interactions permit the nanofiber to slide diffusion along the fiber in either direction that minimizes the exposure of hydrophobic alanines and eventually fill the gaps. The sliding diffusion phenomenon was also proposed for nucleic acids of polyA and polyU in 1956. 30 , 31 For clarity, these β‐sheets are not presented as twisted strands. Image courtesy of Hidenori Yokoi 29
