Fig. 2.
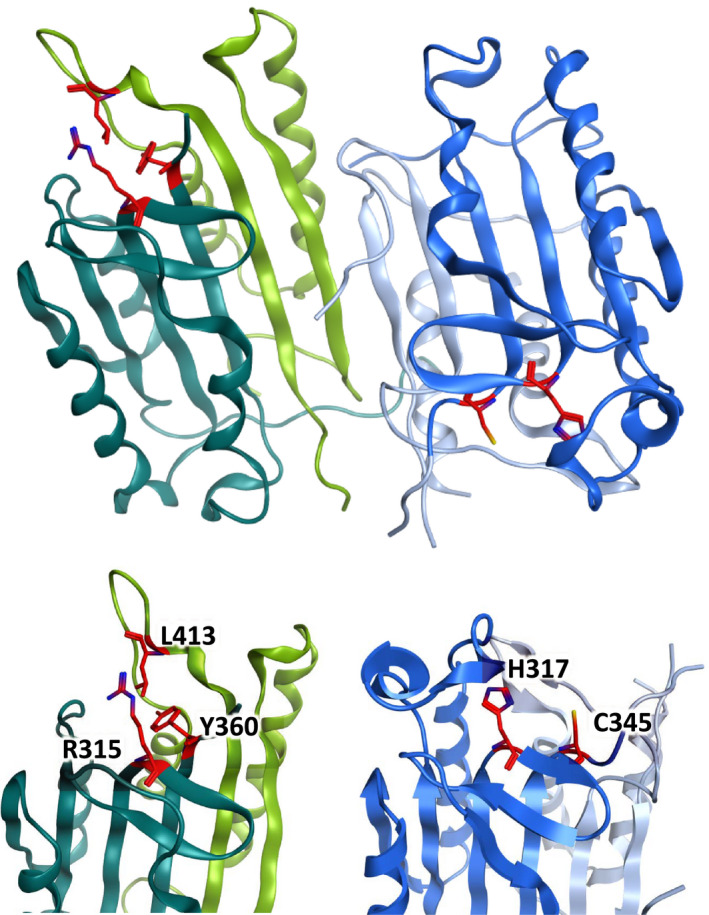
(Upper) Representation of the crystal structure of the caspase‐8 and FLIP(L) heterodimer (PDB 3H13) 33. The formation of the heterodimer between the (pseudo)catalytic domains of FLIP(L) (green) and caspase‐8 (blue) is energetically favorable compared with the caspase‐8 homodimer. This explains why FLIP(L) can promote caspase‐8 activation and apoptosis in certain conditions (see text for details), as when part of a FLIP(L)/caspase‐8 heterodimer, caspase‐8 has restricted enzymatic activity that is capable of efficiently cleaving and processing adjacent procaspase‐8 homodimers, the rate‐limiting step of procaspase‐8 activation. (Lower) The amino acid residues in the active caspase domain of caspase‐8 and the equivalent pseudo‐caspase region of FLIP(L) have been highlighted in red. Notably, the amino acid changes in FLIP(L)’s pseudo‐caspase domain that render it catalytically inactive are evolutionarily conserved, suggestive of important functionality.
