Fig. 3.
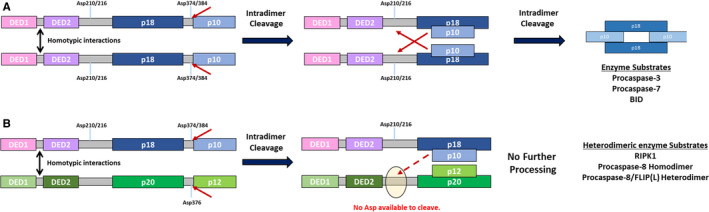
Processing and substrates of procaspase‐8 homodimers and procaspase‐8/FLIP(L) heterodimers. (A) Homodimerization is initially mediated via homotypic DED interactions. Within a homodimer, the caspase‐8 catalytic domains are arranged in an antiparallel fashion. This creates an enzymatic active site, which can then cleave adjacent homodimers in the region between large (p18) and small (p10) catalytic subunits. This cleavage enables the second activation step to take place, which is intradimer cleavage in the linker region between p18 and the DEDs. The p18/p10 heterotetramers that are formed can be released from the complex and activate apoptosis by cleaving procaspases‐3/7 and BID. (B) Formation of a heterodimer between the (pseudo)catalytic regions procaspase‐8 and FLIP(L) is energetically favorable compared with caspase‐8 homodimers. This promotes formation of a FLIP(L)/caspase‐8 heterodimeric enzyme that can efficiently cleave adjacent procaspase‐8 homodimers between their large and small catalytic subunits, thereby promoting processing of these homodimers. The heterodimer also cleaves adjacent heterodimers and RIPK1. The lack of critical cysteine in FLIP(L)’s ‘active site’ and lack of a suitable target site for caspase‐8 in the region between FLIP(L)’s DED2 and p20 subunit prevent intradimer cleavage of the heterodimer, which is therefore retained in the complex.
