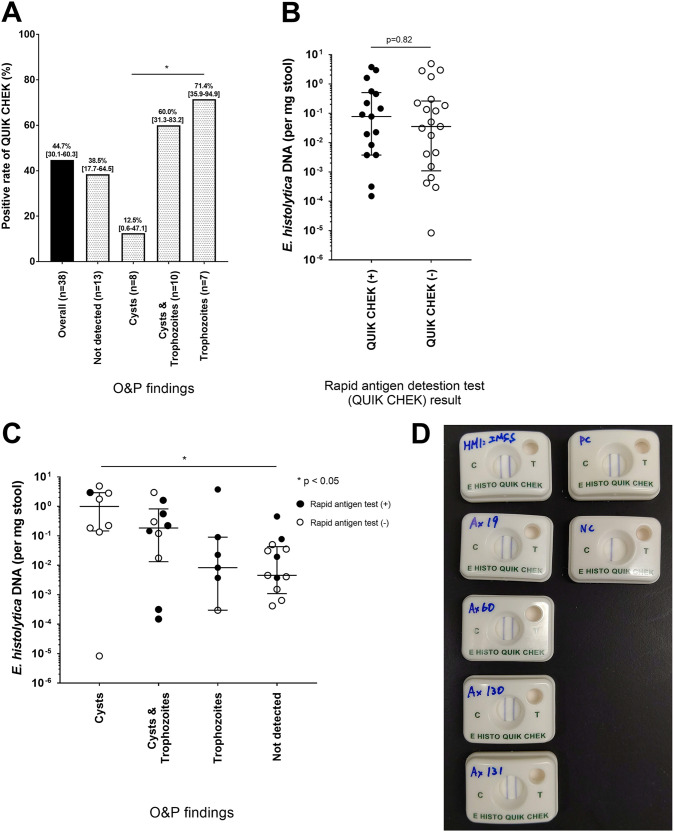
FIG 3.
Factors potentially influencing the diagnostic test outcome. (A) The sensitivity of the rapid antigen detection test (Quik Chek) was compared in accordance with the O&P findings. (B) The pathogen burden in the stool samples was determined by quantitative PCR (qPCR) and compared between the positive and negative stool samples based on the antigen detection test. (C) The pathogen burden quantified by qPCR was compared in accordance with the O&P findings. (D) Quik Chek was applied to in vitro cultured clinical strains using a concentration of 106 trophozoites/ml. HM-1:IMSS, laboratory reference strain; Ax19, clinical strain from Quik Chek-positive liver abscess sample; Ax60, Ax130, and Ax131, clinical strains from Quik Chek-negative stool samples; PC, positive control; NC, negative control; O&P, ova and parasite examination; Quik Chek, rapid antigen detection test (E. histolytica Quik Chek).