Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, critical illness, intubation
Abstract
In critically ill patients with coronavirus disease 2019, there has been considerable debate about when to intubate patients with acute respiratory failure. Early expert recommendations supported early intubation. However, as we learned more about this disease, the risks versus benefits of early intubation are less clear. We report our findings from an observational study aimed to compare the difference in outcomes of critically ill patients with coronavirus disease 2019 who were intubated early versus later in the disease course. Early need for intubation was defined as intubation either at admission or within 2 days of having a documented Fio2 greater than or equal to 0.5. In the final sample of 111 patients, 76 (68%) required early intubation. The mean age among those who received early intubation was significantly higher (69.79 ± 12.15 vs 65.03 ± 8.37 years; p = 0.038). Also, the patients who required early intubation had significantly higher Sequential Organ Failure Assessment scores at admission (6.51 vs 3.48; p ≤ 0.0001). The outcomes were equivocal among both groups. In conclusion, we suggest that the timing of intubation has no impact on clinical outcomes among patients with coronavirus disease 2019 pneumonia.
To the Editor:
Patients with coronavirus disease 2019 (COVID-19) vary from being asymptomatic to experiencing life-threatening critical illness. The basic pathophysiology of severe viral pneumonia is hypoxic respiratory failure secondary to severe acute respiratory distress syndrome (ARDS) (1). Most patients admitted to the ICU end up requiring mechanical ventilation (2, 3). In patients with ARDS, delays in intubation have been associated with higher mortality (4, 5). However, in patients with COVID-19, there has been considerable debate on how to optimize management of acute respiratory failure/ARDS.
Unique to COVID-19, many patients present with significant hypoxia, but only few other signs typically seen in patients with respiratory failure. Although these patients seem quite stable at first, respiratory decompensation occurs frequently. When this does occur, the trajectory is typically quite rapid, making the process of intubation a perilous affair. As a response, several experts from China, Europe, and the United States supported a strategy of intubating patients early under the premise that early intubation allowed for more controlled circumstances and would provide superior lung protection for the patient compared with spontaneous breathing (6, 7). These recommendations led to a rapid rise in ventilator utilization, at one point threatening to overwhelm available mechanical ventilator resources worldwide (8). However, recognition that intubation and mechanical ventilation have inherent risks (ventilator-associated pneumonia, airway injury, ventilator-associated lung injury, and hemodynamic disturbances caused by positive pressure ventilation) has led to a state of relative equipoise about whether the early intubation strategy is indeed better for patients. This has led some experts to recommend abandoning the early intubation strategy (7). Despite these recommendations, the optimal threshold regarding when to intubate patients with COVID-19 pneumonia remains unclear.
We did a retrospective analysis at our institution, aiming to evaluate the association between timing of intubation and outcomes among critically ill patients with COVID-19. A total of 128 ICU patients who tested positive via polymerase chain reaction for COVID-19 were evaluated in our hospital from period of March 15 to May 30, 2020. Eight patients were excluded as they were transferred to an outside hospital or still admitted at the time of data analysis. Patients who did not require intubation were also excluded. In the final sample of 111 patients, 76 (68%) were intubated early in the disease course. Early need for intubation was defined as intubation either at admission or less than 2 days since the onset of increased oxygen requirements—that is the time since the patients require more than 50% of Fio2, that is greater than 10 L nasal cannula or nonrebreather masks or high-flow nasal cannula (HFNC—device capable of delivering up to 100% heated and humidified oxygen at a flow rate of 30–60 L/min) or noninvasive positive pressure ventilation. All intubations greater than or equal to 2 days following the onset of increased oxygen requirements were considered late intubations. This study was approved by the institutional review board at Albert Einstein Medical Center, Philadelphia (IRB-2020-458).
There were no fixed protocols or predefined criterion for intubation. The decision to intubate was left to the discretion of the attending intensivist who responded in accordance with patients’ individual needs and clinical status. Most of the clinicians practiced early intubation strategy during the beginning of the pandemic. However, as more data emerged and recommendations changed (7), clinicians were more comfortable with monitoring patients on noninvasive modes of oxygenation (such as HFNC or noninvasive positive pressure ventilation). The clinician’s judgment to initiate mechanical ventilation was influenced by multitude of factors including oxygen saturation, respiratory rate, work of breathing, mental status, and hemodynamics.
The mean age (± sd) of the sample population was 68 ± 11.28 years. Forty-six percent were female, and 65% were African American. Common chronic comorbidities included hypertension (87%), diabetes mellitus (57%), and chronic obstructive pulmonary disease (14%). Demographic and clinical characteristics are provided in Table 1.
TABLE 1.
Demographics, Clinical Characteristics, and Outcomes of Critically Ill Patients With Coronavirus Disease 2019
| Characteristics | Early (n = 76) | Late (n = 35) | p |
|---|---|---|---|
| Age, mean ± sd | 69.79 ± 12.15 | 65.03 ± 8.37 | 0.038 |
| Female gender, n (%) | 34 (45) | 17 (49) | 0.838 |
| Ethnicity, n (%) | 0.885 | ||
| African American | 48 (63) | 24 (69) | |
| Caucasian | 4 (5) | 2 (6) | |
| Hispanic | 9 (12) | 4 (11) | |
| Other | 13 (17) | 5 (14) | |
| Unknown | 2 (3) | 0 (0) | |
| Comorbidities n (%) | |||
| Body mass index, mean ± sd | 30.27 ± 8.07 | 32.17 ± 7.81 | 0.247 |
| Chronic obstructive pulmonary disease | 10 (13) | 6 (17) | 0.573 |
| Asthma | 5 (7) | 4 (11) | 0.459 |
| Obstructive sleep apnea | 10 (13) | 3 (9) | 0.752 |
| Heart failure | 15 (20) | 9 (26) | 0.469 |
| Atrial fibrillation | 12 (16) | 2 (6) | 0.218 |
| Liver cirrhosis | 2 (3) | 3 (9) | 0.323 |
| Diabetes | 45 (59) | 18 (51) | 0.537 |
| Chronic kidney disease | 14 (18) | 7 (20) | 1.000 |
| End stage renal disease on dialysis | 8 (11) | 2 (6) | 0.500 |
| HIV | 1 (1) | 0 (0) | 1.000 |
| Coronary artery disease | 18 (24) | 10 (29) | 0.641 |
| Hypertension | 66 (87) | 31 (89) | 1.000 |
| Laboratory/variables at admission | |||
| Fio2% on intubation, mean ± sd | 90.30 ± 18.08 | 97.94 ± 5.91 | 0.001 |
| Pao2:Fio2 ratio 0 hr intubation, median (IQR) | 106 (63–169) | 111 (60–178) | 0.644 |
| Serum ferritin, median (IQR) | 1,262 (560–2,829) | 824 (468–1,483) | 0.070 |
| Peak ferritin, median (IQR) | 2,312 (840–5,240) | 1,981 (807–4,633) | 0.850 |
| d-dimer, median (IQR) | 2,805 (1,418–5,970) | 2,560 (1,160–4,130) | 0.333 |
| Peak d-dimer, median (IQR) | 5,990 (2,945–18,998) | 7,590 (7,590–15,235) | 0.524 |
| CRP, median (IQR) | 180 (102–255) | 133 (81–236) | 0.320 |
| Peak CRP, median (IQR) | 218 (155–305) | 227 (165–301) | 0.962 |
| Procalcitonin, median (IQR) | 0.62 (0.22–2.48) | 0.35 (0.11–1.01) | 0.088 |
| Peak procalcitonin, median (IQR) | 1.52 (0.36–10.64) | 0.65 (0.17–9.59) | 0.139 |
| LDH, median (IQR) | 505 (363–656) | 456 (394–586) | 0.588 |
| Peak LDH, median (IQR) | 662 (522–1,046) | 720 (555–857) | 0.933 |
| Coronavirus disease 2019 treatment, n (%) | |||
| Hydroxychloroquine | 49 (65) | 27 (77) | 0.197 |
| Steroids | 56 (74) | 25 (71) | 0.821 |
| Tocilizumab | 23 (30) | 11 (31) | 1.000 |
| Remdesivir | 11 (15) | 5 (14) | 1.000 |
| Proning | 23 (30) | 15 (44) | 0.194 |
| Paralytics | 28 (37) | 16 (47) | 0.437 |
| Clinical outcomes | |||
| Days prior intubation median (IQR) | 1 (0–2) | 4.5 (3–7) | < 0.0001 |
| Days on high flow/non-invasive ventilation/elevated O2 requirements prior to intubation, median (IQR) | 0.38 (0–1) | 3 (2.5–5.0) | < 0.0001 |
| SOFA at admission, mean ± sd | 6.51 ± 3.48 | 3.74 ± 2.41 | < 0.0001 |
| SOFA ICU admission, mean ± sd | 8.15 ± 3.29 | 6.29 ± 2.80 | 0.005 |
| Inpatient death, n (%) | 54 (71) | 20 (57) | 0.194 |
| Need for continuous renal replacement therapy/hemodialysis, n (%) | 23 (30) | 7 (20) | 0.358 |
| Reintubation, n (%) | 6 (10) | 4 (15) | 0.488 |
| Days on ventilator, mean ± sd | 10.41 ± 7.53 | 8.00 ± 7.82 | 0.125 |
| ICU length of stay, mean ± sd | 10.76 ± 7.59 | 8.19 ± 7.53 | 0.103 |
| Hospital length of stay, mean ± sd | 13.98 ± 8.71 | 15.15 ± 8.11 | 0.509 |
CRP = C-reactive protein, IQR = interquartile range, LDH = lactate dehydrogenase, SOFA = Sequential Organ Failure Assessment.
The mean age among those who required early intubation was significantly higher compared with those who underwent late intubation (69.79 ± 12.15 vs 65.03 ± 8.37 yr; p = 0.038). There were no other significant demographic differences between the two groups. Inflammatory markers such as serum ferritin, d-dimer, CRP, procalcitonin, and LDH were similar between the two groups. There was no difference in the Pao2:Fio2 ratio between groups at the time of intubation. There were no significant differences in COVID-19 specific treatments such as hydroxychloroquine, steroids, tocilizumab, or, remdesivir between groups. The rates of early intubations remained high throughout the study period with a slow decline in the end. An opposite trend was observed in number of late intubations (Fig. 1). This was in accordance with the change in intubation practices as described above.
Figure 1.
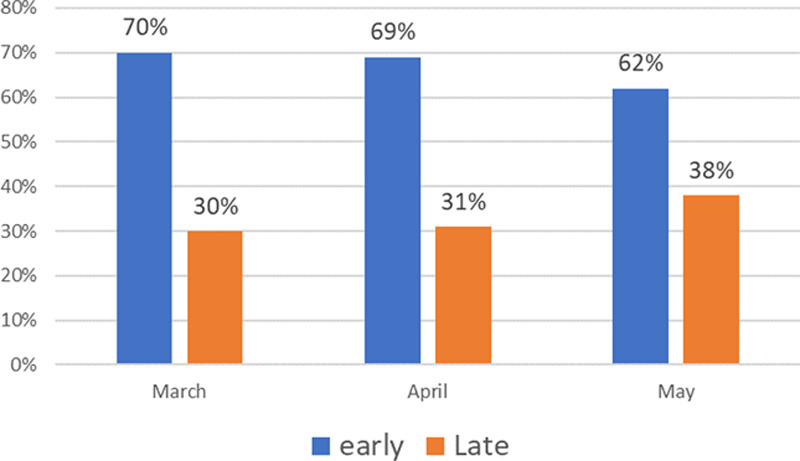
Trend of early and late intubations during each month of the study period.
Prior studies have shown that age is an important predictor of mortality (5, 6). Under these circumstances, it would seem plausible that mortality might be higher in those who were intubated early. Although there was a trend toward increased mortality (71% vs 57%; p = 0.194) and increased days on a ventilator (10.41 ± 7.53 vs 8.00 ± 7.82; p = 0.125) among patients who required early intubation compared with those who underwent late intubation, this was not statistically significant.
The trajectory of decline in clinical status was rather rapid among patients requiring early intubation. This was evident from the fact that the median days to intubation were significantly lower in patients who were intubated early compared with those intubated late (1 vs 4.5 d; p < 0.001). Time that the patients spent on more than 50% of Fio2 (> 10 L nasal cannula or nonrebreather masks or HFNC or noninvasive positive pressure ventilation) was also significantly lower among patients requiring early intubation (0.38 d vs 3 d; p < 0.001).
With respect to respiratory mechanics, there was a significantly higher mean compliance in patients who required early intubation at the 24- and 48-hour time periods (32 vs 27 mL/cm H2O; p = 0.016) and (33 vs 25 mL/cm H2O; p < 0.0001),respectively (Fig. 2). There was also a significantly lower plateau pressure at less than 24-hour time period among patients undergoing early intubation (25 vs 27 cm H2O; p = 0.024) (Fig. 2). The respiratory compliance noted in both the groups was comparable with those reported in earlier cohorts (2, 9, 10). This variability in respiratory mechanics suggests a significant heterogeneity in the disease process as described earlier by Gattinoni et al (11), although the lung compliance in their group was much higher (9). They categorized the patients with COVID-19 pneumonia in two different phenotypes—phenotype L characterized by low elastance (high compliance) and phenotype H characterized by high elastance (low compliance). Although both phenotypes have been observed, the clinical significance of having one phenotype versus another is not well studied. Both groups in our cohort were noted to have similar Pao2:Fio2 ratio suggesting that the extent of lung injury was similar between groups independent of whether lung compliance or plateau pressure was similar. In this case, the absence of an outcome difference between groups in this setting at worst supports that delaying intubation was not harmful to patients when compared with early intubation.
Figure 2.
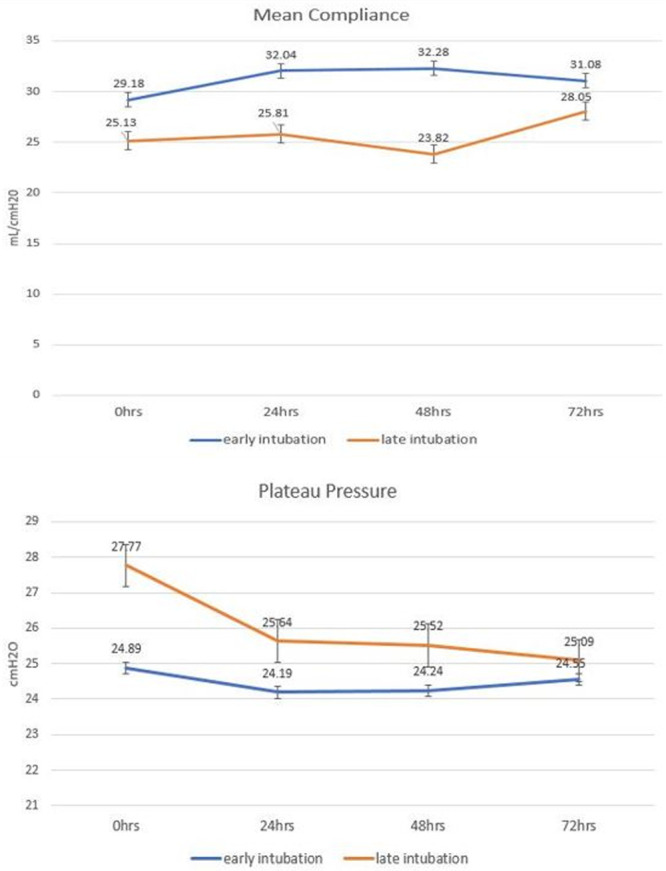
Mean static compliance and plateau pressures after 0, 24, 48, and 72 hr of intubation.
Sequential Organ Failure Assessment (SOFA) score has been associated with higher mortality among patients admitted to the ICU (10). In our study, the patients who required early intubation had a significantly higher SOFA score on hospital admission and ICU admission when compared with the late intubation group (6.51 vs 3.48; p ≤ 0.0001) and (8.15 vs 6.29 p = 0.005), respectively. However, no difference in mortality was noted among the two groups.
We acknowledge that there are several limitations to our study. First, this study is a single-center observational study with a relatively small cohort of patients. The decision to intubate was left to the discretion of the ICU team, and we did not have a predefined criterion for intubation specifically for patients with COVID-19 pneumonia. Nonetheless, the analysis provides an early, pragmatic evaluation of outcomes associated with COVID-19–associated respiratory failure and intubation timing. The factors highlighted in our study will provide some guidance for future prospective study planning.
In conclusion, we suggest that the timing of intubation does not seem to be significantly associated with poor clinical outcomes in critically ill patients with COVID-19. The timing of intubation seems to be driven mainly by disease severity and rate of progression. Hence, trial of noninvasive strategies of oxygenation in an attempt to avoiding intubation might not be harmful. However, larger prospective studies are needed to fully elucidate these effects (Table 2, Supplemental Digital Content 1, http://links.lww.com/CCX/A390)
Supplementary Material
Atul Matta, MD, Siddique Chaudhary, MD, Department of Pulmonary Critical Care and Sleep Medicine, Einstein Medical Center, Philadelphia, PA; Kevin Bryan Lo, MD, Robert DeJoy III, DO, Fahad Gul, MD, Ricardo Torres, MD, Department of Medicine, Einstein Medical Center, Philadelphia, PA; Neal Chaisson, MD, Department of Pulmonary and Critical Care Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, OH; Gabriel Patarroyo-Aponte, MD, Department of Pulmonary Critical Care and Sleep Medicine, Einstein Medical Center, Philadelphia, PA, and Department of Medicine, Einstein Medical Center, Philadelphia, PA, and Department of Medicine, Sidney Kimmel College of Thomas Jefferson University, Philadelphia, PA
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The study was approved by Institutional Review Board at Albert Einstein Medical Center—Philadelphia.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zanella A. Critically ill patients with COVID-19 in New York city. Lancet. 2020; 395:1740–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer PR, Gajic O, Nanchal R, et al. ; ICON Investigators (Supplemental Appendix 1) ICON Investigators (Supplemental Appendix 1). Association between timing of intubation and outcome in critically ill patients: A secondary analysis of the ICON audit. J Crit Care. 2017; 42:1–5 [DOI] [PubMed] [Google Scholar]
- 5.Kangelaris KN, Ware LB, Wang CY, et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med. 2016; 44:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo MZ, Huang YG, Ma WH, et al. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin Med Sci J. 2020; 35:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rola P, Farkas J, Spiegel R, et al. Rethinking the early intubation paradigm of COVID-19: Time to change gears? Clin Exp Emerg Med. 2020; 7:78–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliff S, Satariano A, Silver-greenberg J, et al. There Aren,t Enough Ventilators to Cope with the Coronavirus. The New York Times. 2020. March 18 Retrieved from https://www.nytimes.com/2020/03/18/business/coronavirus-ventilator-shortage.html. Accessed July 21, 2020 [Google Scholar]
- 9.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: A cohort study. Am J Respir Crit Care Med. 2020; 201:1560–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenck EJ, Hoffman K, Goyal P, et al. Respiratory mechanics and gas exchange in COVID-19 associated respiratory failure. Ann Am Thorac Soc. 2020; 17:1158–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020; 46:1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001; 286:1754–1758 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


