FIG 5.
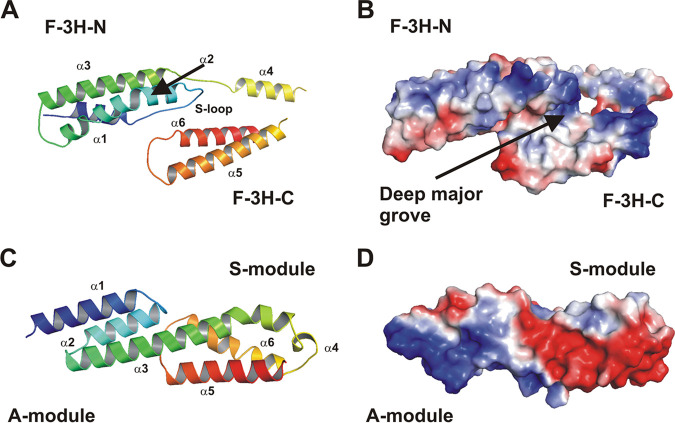
Structural analysis of Embp. (A) Cartoon plot of the F-repeat showing the three helix-bundle arrangement. N-terminal helix bundle F-3H-N (aa 2569 to 2652) and C-terminal helix bundle F-3H-C (aa 2660–2738) are connected by a short linear linker (L2, aa 2653 to 2659). Within F-3H-N, helix α1 and α2 are connected by a Sandwich-loop (S-loop) which potentially is of fundamental importance for the structural integrity of the F-repeat as a compact unit. Helix α2 shows a remarkable almost 45° kink at residue Ala-2607. The third helix α3 (aa 2629 to 2652) follows in the opposite direction after a short loop (L1, aa 2624 to 2628). Helix α3 is slightly bended to allow a tight interaction between all three helices. Within F-3H-C, the first helix α4 (aa 2660 to 2673) is followed by the second helix α5 (aa 2685 to 2709). The connecting loop L3 (aa 2674 to 2684) is distorted and not defined by the electron density. Helix α5 (aa 2685 to 2709) and helix α6 (aa 2720 to 2738) are connected by loop L4 (aa 2710 to 2719). The model is colored according to the sequence, from blue at the N terminus to red at the C terminus. The other structural elements are assigned accordingly. (B) An electrostatic surface potential representation in the same orientation as in panel A reveals a prominent deep major groove flanked by helix α2 (from F-3H-N) and helix α4, α5, and α6 (from F-3H-C). (C) Cartoon plot of the FG-repeat showing the three helix-bundle arrangement composed of an A- and S-module. At the junction between the A- and S-module, the completely conserved residues, Leu-6798, Gln-6802, and Leu-6829 (from A-module), create a hydrophobic core with highly conserved hydrophobic residues, Met-6833, Ile-6882, and Ile-6887 (from S-module), which are further stabilized by a direct hydrogen bond between Gln-6802 and Ile-6882. Therefore, both three-helix bundles of the FG-repeat are connected rather tightly. Helix α4 is running perpendicular to all other helices and connects helix α3 and helix α5. These structural features are consistent with those described for EbhA-R7-R8 (29) from S. aureus Ebh. The model is colored according to the sequence, from blue at the N terminus to red at the C terminus. The central long helix α3 connects the A- and S-module and is colored in green. (D) An electrostatic surface potential representation in the same orientation as in panel C reveals a remarkable strong dipole character of the FG-repeat. The N-terminal region of the A-module is strongly positively charged, whereas the C-terminal region (S-module) is strongly negatively charged.