FIG 8.
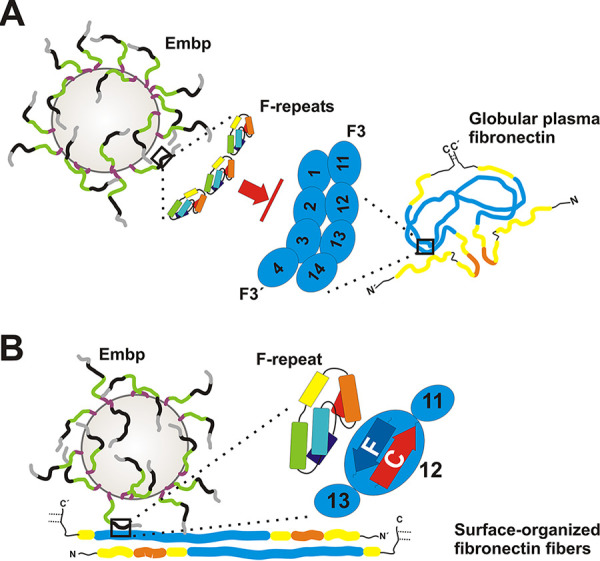
Graphical summary of Embp-mediated S. epidermidis interactions with fibronectin. S. epidermidis displays FG-repeat (green)- and F-repeat (black)-containing Embp on the cell surface of S. epidermidis (4), according to SAXS data most likely organized in elongated fibers. (A) Embp-mediated S. epidermidis interactions with globular Fn. The compact globular architecture of the soluble Fn-dimer is stabilized via intermolecular interactions, essentially involving FN2-3 of one Fn molecule (zoom-in right; F3′) and FN12 of the second molecule (zoom-in right, F3) (33). F-repeats (zoom-in left) or FG-repeats (not shown) possess Fn-binding activity through interactions with FN12. F-repeat and FG-repeat binding sites in FN12 are blocked by intramolecular Fn-Fn interactions, preventing Embp-mediated binding to globular Fn and its recruitment to the bacterial cell surface. (B) Embp-mediated S. epidermidis interactions with immobilized Fn. During surface deposition, Fn dimers become elongated by resolving intramolecular interactions and additional structural rearrangements of the F3 domain (73). As a consequence, F-repeat and FG-repeat binding sites within FN12 become accessible (i.e., within β-strands C and F; zoom-in), thus allowing S. epidermidis to adhere to Fn-conditioned surfaces. This process is fostered by additional, as-yet-uncharacterized S. epidermidis interactions with Fn. The figure is not drawn to scale.
