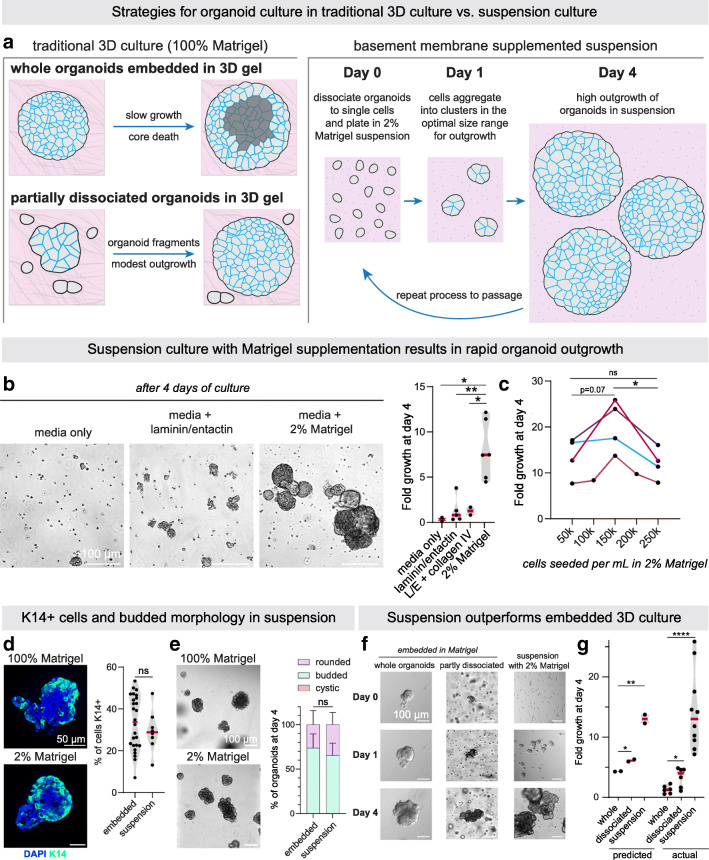
Fig. 2.
Suspension culture supplemented with basement membrane-rich extract facilitates rapid expansion of tumor organoids. a Schematic of organoid culture methods. Traditionally, organoids are either plated intact in 3D gels or briefly mechanically or enzymatically disrupted to generate organoid fragments, as well as debris and single cells, before plating in 3D culture. Right, we present an alternative culture method where single tumor cells are aggregated in non-adherent suspension supplemented with basement membrane-rich extract to form multicellular aggregates optimally sized for rapid outgrowth. b DIC images (left) and fold growth by viable cell number (right), of MMTV-PyMT organoids after 4 days in organoid media only, media supplemented with 2% Matrigel, media +100 μg/mL laminin/entactin, or media +70 μg/mL laminin/entactin +30 μg/mL collagen IV. Each dot is a biological replicate. P values = unpaired t-tests. c To determine the optimal density to seed MMTV-PyMT single cells for aggregation in 2% Matrigel suspension culture, cells were plated at 0.5 × 105 to 2.5 × 105 viable cells/mL to form small clusters. After 4 days, fold-growth by viable cell number was measured. n = 4 mice, each line is a biological replicate. P values = paired t-tests. d Left, Keratin-14 immunofluorescence images of MMTV-PyMT organoids cultured embedded in 100% Matrigel vs. cultured in suspension supplemented with 2% Matrigel. Right, quantification of the % of cells K14+ (basal). n = 2 mice (suspension), n = 3 mice (embedded), n = 4161 cells analyzed from 34 organoids. P-value = unpaired t-test. e Images of organoids after 4 days in culture embedded in 100% Matrigel or seeded in suspension culture supplemented with 2% Matrigel. Right, summary of organoid morphology at D4. “Rounded” refers to organoids with smooth spherical borders, vs. “budded” organoids with multicellular budded protrusions. No organoids scored had hollow, cystic morphology in either condition. n = 3 mice, n = 285 organoids in suspension, 259 organoids embedded in 100% Matrigel. P-value = unpaired t-test. f DIC images of MMTV-PyMT organoids 0 days, 1 day, and 4 days after initial plating in the methods described in (a). Whole organoids or briefly dissociated (5 minutes Accumax treatment) organoids were plated in 3D matrigel, or single cells were allowed to aggregated at 150,000 viable cells/mL in organoid media +2% Matrigel. g From the area measurements of Fig. 2f (see Table 2) and the growth parameters identified in Fig. 1f, estimated fold growth for each culture method was predicted (see Methods section for piecewise function). Then actual fold growth of MMTV-PyMT organoids by viable cell number after 4 days in each of the 3 culture methods was measured. Each dot is a mouse. P-values = unpaired t-tests