Abstract
This study investigated the effects of encapsulated cinnamaldehyde (CIN) and citral (CIT) alone or in combination (CIN + CIT) on the growth performance and cecal microbiota of nonvaccinated broilers and broilers vaccinated against coccidiosis. Vaccinated (1,600) and nonvaccinated (1,600) 0-day-old male Cobb500 broilers were randomly allocated to 5 treatments: basal diet (control) and basal diet supplemented with bacitracin (BAC, 55 ppm), CIN (100 ppm), CIT (100 ppm), and CIN (100 ppm) + CIT (100 ppm). In general, body weight (BW) and feed conversion ratio were significantly improved in birds treated with BAC, CIN, CIT, and CIN + CIT (P < 0.05) but were all decreased in vaccinated birds compared with nonvaccinated birds (P < 0.05). Significant interactions (P < 0.05) between vaccination and treatments for average daily gain during the periods of starter (day 0–9) and BW on day 10 were noted. Broilers receiving vaccines (P < 0.01) or feed supplemented with BAC, CIN, CIT, or CIN + CIT (P < 0.01) showed reductions in mortality rate from day 0 to 28. The incidences of minor coccidiosis were higher (P < 0.05) in vaccinated birds than in nonvaccinated birds. Diet supplementation with BAC or tested encapsulated essential oils showed comparable effects on the coccidiosis incidences. Similar to BAC, CIN and its combination with CIT reduced both incidence and severity of necrotic enteritis (P < 0.05). No treatment effects were observed on the cecal microbiota at the phyla level. At the genus level, significant differences between vaccination and treatment groups were observed for 5 (Lactobacillus, Ruminococcus, Faecalibacterium, Enterococcus, and Clostridium) of 40 detected genera (P < 0.05). The genus Lactobacillus was more abundant in broilers fed with CIT, while Clostridium and Enterococcus were less abundant in broilers fed with CIN, CIT, or CIN + CIT in both the vaccinated and nonvaccinated groups. Results from this study suggested that CIN alone or in combination with CIT in feed could improve chicken growth performance to the level comparable with BAC and alter cecal microbiota composition.
Key words: broiler, cecal microbiota, coccidiosis, essential oil, growth performance
Introduction
Intestinal diseases such as coccidiosis and necrotic enteritis (NE) are responsible for immense economic losses (several billion USD annually) to the poultry industry worldwide (Dalloul and Lillehoj, 2006). The common Eimeria species involved in coccidiosis include Eimeria tenella, Eimeria maxima, and Eimeria acervulina. These species can multiply and damage the intestine epithelial layer of the chicken duodenum, mid-intestine, and ceca, reducing feed intake and nutrient digestibility (Long and Jeffers, 1986, Martin et al., 1997, Dahiya et al., 2006). The NE caused by Clostridium perfringens is an enteric disease characterized by severe necrosis of intestinal mucosa, which impairs broiler productivity (McDevitt et al., 2006). Necrotic enteritis B-like toxin produced by C. perfringens is considered the major toxin responsible for NE (Keyburn et al., 2008), although many other toxins such as Beta 2, Tpel, and virulent factors also play a role (McDonel, 1980, Keyburn et al., 2008, Lepp et al., 2013, Prescott et al., 2016). Traditionally, coccidiosis and NE have been effectively controlled by the application of antimicrobials in feed (Reid, 1990). However, owing to the misuse and overuse of antimicrobials, resistance to antibiotics has become a public health issue, leading to new challenges to urgently develop effective alternatives to antibiotics to control enteric diseases in broilers (Casewell et al., 2003, Agyare et al., 2018). Recently, the Chicken Farmers of Canada revised its antimicrobial use to eliminate the preventive use of category II antibiotics in 2018 and that of category III antibiotics by the end of 2020 (Chicken Farmers of Canada, 2019).
To overcome the potential increase in mortality and morbidity of broilers due to the ban of in-feed antimicrobial use, probiotics, prebiotics, organic acid, essential oils (EOs), and vaccines are becoming studied as the alternatives to antibiotics (Griggs and Jacob, 2005, Diarra et al., 2007, Osman and Elhariri, 2013). In the 1970s, coccidiosis vaccines containing mixtures of living Eimeria species were introduced to control coccidiosis by stimulating intestinal immune T-cells of broilers (Lillehoj and Lillehoj, 2000, Wallach, 2010). The EOs are aromatic compounds derived from plants, and many are potent inhibitors of bacterial growth (Si et al., 2006, Bakkali et al., 2008). Natural EOs are extracted from parts of trees such as leaves, roots, and barks. Synthetic EOs may contain toxins although the purity could reach more than 95%. Although no studies have been conducted to compare the effects of natural and synthetic EOs on the performance of poultry, cinnamaldehyde (CIN) used in the present study was synthetic while citral (CIT) was a natural EO.
CIT and cinnamon oil are 2 EOs that have been used in medication over the last century (Burt, 2004). CIN compounds displaying a pale-yellow color are extracted from the bark of cinnamon trees or from other species of the genus Cinnamomum (Tisserand and Balacs, 1995). Cinnamon oil has antimicrobial effects but has additionally been used for food flavoring in sweets and chewing gum (Nabavi et al., 2015). It has been demonstrated that cinnamon powder in feed could improve meat quality and growth quality of broiler chickens (Sang-Oh et al., 2013) and alleviate intestinal injury (Wang et al., 2015). CIT (3,7-dimethyl-2-6-octadienal) is extracted from different plants including lemongrass (around 76% by gas chromatography) (Silva et al., 2008), lemon myrtle (>90%) (Tisserand and Balacs, 1995), and Lindera citriodora (about 65%) (Ohtsuru et al., 1967). The antimicrobial activities of CIT have been documented as effective against several bacterial pathogens including C. perfringens (Onawunmi, 1989, Si et al., 2006, Si et al., 2009, Yang et al., 2016). Previous studies have demonstrated that protection is required for effective delivery of EOs to the animal gut (Zhang et al., 2014, Zhang et al., 2015, Ma et al., 2016, Yang et al., 2015a, Omonijo et al., 2017). The use of CIT and cinnamon in feed as antibiotic alternatives could be limited because of physical and chemical instabilities during storage and in the gastrointestinal tract of poultry (Kimura et al., 1981, Tian et al., 2016). A recent study indicated that the incorporation of a soy protein-polysaccharide Maillard reaction product stabilized CIT and offered protection to CIT during the storage, upon low pH in the stimulated gastrointestinal tract fluid and heat treatment (Yang et al., 2015b). The protection could be due to the incorporation of soy protein-polysaccharide Maillard reaction product that may have shield peptide bonds against proteolysis and thus retard the release of CIT from the droplets (Yang et al., 2016).
In this study, the effectiveness of encapsulated CIT and cinnamaldhyde alone or in combination in feed on growth performance, gut health, and cecal microbiota was evaluated in broilers vaccinated or not against coccidiosis.
Materials and methods
Essential Oils
CIT (a mixture of cis and trans isomers, 95% purity) and CIN (≥95% purity) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). The CIN and CIT were encapsulated separately using the materials and methods described previously with minor modifications (Yang et al., 2015b, Ma et al., 2016).
Experimental Design
A total of 3,200 0-day-old male Cobb 500 broiler chickens were housed in 40 floor pens (80 birds per pen). The pens were assigned to 2 groups, half of the birds (1,600) received a commercial coccidiosis vaccine via spraying at 0-day of age and the other half (1,600) did not receive the vaccine. Pens in each of these 2 groups were randomly allocated to 5 dietary treatments (4 pens per treatment) in a complete randomized design. The dietary treatments were 1) basal diet (enrich with animal by-products) serving as the control; 2) basal diet with 55 mg/kg BAC (positive control); 3) basal diet with 100 mg/kg encapsulated CIN; 4) basal diet with 100 mg/kg encapsulated CIT; and 5) basal diet with a combination of 100 mg/kg encapsulated CIN and 100 mg/kg CIT (CIN + CIT). Broilers were fed a starter diet from the age of 0 to 9 D, grower diet from age 10 to 19 D, and a finisher diet from age 20 to 28 D. The EOs were fed from day 10 to 28 (grower, finisher), and BAC was fed from day 0 to 28 (starter, grower, and finisher). The starter, grower, and finisher diets (Table 1) were formulated and pelleted with wheat and corn as the principal cereals and soybean meal, fish meal, and meat meal as protein sources according to the nutritional recommendation by Cobb 500 (2012). No coccidiostats were provided in the diets to prevent coccidiosis.
Table 1.
Feed ingredients and nutrient composition for starter (days 0–9), grower (days 10–19), and finisher (days 20–28) of chicken (%, as feed basis otherwise indicated).
| Ingredients | Inclusion in basal diet |
||
|---|---|---|---|
| Starter | Grower | Finisher | |
| Wheat | 30.00 | 35.00 | 35.00 |
| Soybean meal | 27.79 | 21.89 | 16.76 |
| Vegetable oil | 3.74 | 3.54 | 4.41 |
| Corn | 23.39 | 24.81 | 29.15 |
| Corn gluten meal | 5.00 | 5.00 | 5.00 |
| Limestone | 0.96 | 0.96 | 0.92 |
| Biofos1 | 0.31 | 0.11 | 0.00 |
| Mineral and vitamin Mix2 | 0.25 | 0.25 | 0.25 |
| L-Lysine HCl | 0.12 | 0.13 | 0.25 |
| Sodium chloride | 0.10 | 0.10 | 0.10 |
| DL-Methionine | 0.23 | 0.21 | 0.19 |
| Avizyme 15023 | 0.05 | 0.05 | 0.05 |
| Sodium Bicarbonate | 0.44 | 0.34 | 0.31 |
| Phytase4 | 0.01 | 0.01 | 0.01 |
| Choline | 0.12 | 0.10 | 0.10 |
| Fish meal | 4.50 | 4.50 | 4.50 |
| Meat meal | 3.00 | 3.00 | 3.00 |
| Calculated Nutrients | |||
| Crude Protein | 25.30 | 23.30 | 21.30 |
| Methionine | 0.62 | 0.58 | 0.53 |
| Methionine & Cysteine | 1.08 | 1.01 | 0.94 |
| Lysine | 1.37 | 1.23 | 1.19 |
| Metabolisable Energy, kcal/kg | 3,022 | 3,063 | 3,152 |
| Crude Fat | 6.19 | 6.06 | 7.00 |
| Crude Fiber | 2.31 | 2.24 | 2.11 |
| Calcium | 0.90 | 0.85 | 0.80 |
| Total Phosphorus | 0.66 | 0.60 | 0.55 |
| Available Phosphorus | 0.40 | 0.35 | 0.32 |
| Sodium | 0.20 | 0.17 | 0.16 |
Feed-grade monocalcium phosphate.
Supplied per kilogram of diet: vitamin A, 9,000 IU; cholecalciferol, 5,000 IU; vitamin E, 30 IU; vitamin K, 0.5 mg; cobalamin, 0.007 mg; thiamine, 0.4 mg; riboflavin,6 mg; folicacid,1 mg; biotin,0.15 mg; niacin, 135 mg; pyridoxine, 4 mg; Fe, 125 mg; Mn, 60 mg; Cu, 5 mg; Se, 0.10 mg; I, 0.35 mg; Zn, 50 mg.
Multi-Enzyme System for Wheat-Based Poultry Feed (Halchemix Canada Inc., Toronto, ON, Canada) containing 600 U/g of xylanase, 8,000 U/g of protease, and 800 U/g of amylase.
Ronozyme P5000 (DSM Nutritional Products Canada Inc., Ayr, ON, Canada).
Animals and Management
All experimental procedures performed in this study were approved by the Animal Care Committee of the Centre de recherche en sciences animales de Deschambault (CRSAD; Deschambault, QC, Canada) according to guidelines from the Canadian Council on Animal Care (1993; Canadian Council on Animal Care, Ottawa, ON Canada).
The clean and disinfected wood floor was covered with approximately 3 in. (7.6 cm) of clean softwood shavings, and the bird density was approximately 0.087 m2 per bird. Ventilation was provided by negative pressure with fans. The heat was provided by gas-fired brooders in each pen; water and feed were offered ad libitum through nipple drinkers and tube feeders, respectively, throughout the entirety of the experiment. The temperature was set at 33°C on day 0 and then was reduced by 2.5°C each week. Chicks were exposed to light for 24 h for the first day, 20 h for the second and until day 9, then 18 h thereafter. Birds were fed ad libitum with free access to water throughout the whole experiment. Birds were weighed at the start of the trial (day 0); body weight (BW) and feed intake were measured on day 10, 20, and 28 from each pen, and average daily feed intake (ADFI) and feed conversion ratio (FCR) were calculated. Birds were inspected at least twice per day, and mortalities or culls were removed and necropsied by the “Services Vétérinaires Ambulatoires Triple-V Inc.” (Acton Vale, QC, Canada). The mortality rate was calculated based on the average mortality in each pen on day 0 to 28.
General and Gut Health
Fecal samples were collected on days 6, 9, 13, 16, 20, 23, and 27 from each pen (3/pen; 60/vaccination group/sampling time for a total of 420 samples) for oocyst counts (log10/g). At day 21–22, 4 birds/pen (80 per vaccinated or nonvaccinated group for a total of 160 birds) were sacrificed for necropsy by the “Services Vétérinaires Ambulatoires Triple-V Inc.” The intestines of all sacrificed birds (4 birds per pen: 16/treatment) were examined for evidence coccidiosis and NE. The intestinal health was scored for NE and coccidiosis lesions according to the study by Collier et al., (2003). Intestines were longitudinally opened to score mucosa on a scale of 0 to 3 for NE lesions for each of the upper gut and lower gut (including ceca). Coccidiosis lesions were scored on a scale of 0 to 4 for each of E. maxima which induces bleeding in the middle of the small intestines, mucosa, E. tenella causing severe inflammation of ceca and E. acervulina causing white plaques in the duodenum. The body weights of killed birds were determined.
Genomic DNA Isolation and 16S Ribosomal RNA Gene Sequencing
Cecal contents of the aforementioned 160 sacrificed birds (4 birds/treatment for a total of 40 pooled contents) were used for genomic DNA extraction. Genomic DNA was extracted from frozen cecal content using the QIAamp DNA Stool Mini Kit (QIAGEN, Toronto, Canada) according to the manufacturer's instructions. The purity and concentrations of the extracted DNA were determined using an Invitrogen Qubit 2.0 Fluorometer (Life Technologies Inc., Carlsbad, CA). Sequencing libraries of the 16S ribosomal RNA gene (rRNA) were prepared according to the Illumina 16S Metagenomic Sequencing Library Preparation Guide Rev. B and sequenced on a MiSeq instrument (Illumina). Briefly, a 444-bp fragment spanning the V3-V4 hypervariable region (Escherichia coli 16S rRNA position 340 - 784) was amplified with primers Bakt_341F (5′-CCTACGGGNGGCWGCAG-3′) and Bakt_805R (5′-GACTACHVGGGTATCTAATCC-3′) (Klindworth et al., 2013) containing 5′ Illumina overhang adapter sequences (5′-TCGTCGGCAG CGTCAGATGTGTATAAGAGACAG-3′ and 5′-GTCTCGTGGGCTCGGA GATGTGTATA AGAGACAG-3′, respectively) using 2x KAPA HiFi HotStart ReadyMix (VWR, CA89125-042) and purified with AMPure XP beads (Beckman Coulter A63880). Unique 8-bp dual indexes were added by PCR using the Nextera XT Index Kit (Illumina Inc., FC-131-1002), and PCR products were cleaned up with AMPure XP beads. Samples were pooled together at equimolar concentrations and sequenced using a 600-cycle v3 reagent kit (Illumina, MS-102-3003).
The sequencing data were analyzed by Quantitative Insights Into Microbial Ecology (QIIME, version 1.9.1; Caporaso et al., 2010). Paired-end reads were joined with fastq-join (Aronesty, 2011), and quality filtered and demultiplexed in QIIME using default settings. The reads were clustered at 97% sequence identity (similar as the species level) using uclust (Edgar, 2010), and operational taxonomy units (OTUs) were picked against the Greengenes database (gg_otus_13_8) using an open-reference approach (DeSantis et al., 2006). Taxonomic assignment of the sequences was performed using the uclust consensus taxonomy assigner. Taxa that could not be assigned were presented as 'unclassified' using the highest taxonomic level that could be assigned to them. The sequences were aligned against the Greengenes core set with PyNast (Caporaso et al., 2010), and a phylogenetic tree was constructed with FastTree (Price et al., 2009). Alpha-diversity (within groups) metrics were then calculated by QIIME, and a beta diversity (between groups) distance matrix based on unweighted UniFrac metric (Lozupone and Knight, 2005) was calculated, which was used for principal co-ordinate analysis (PCoA).
Statistical Analysis
The experiment was arranged as 2 × 5 factorial design with 2 groups (vaccinated or nonvaccinated) and 5 feeding treatments (Control, BAC, CIN, CIT, CIN + CIT). Effects of vaccination and feeding treatments on growth performance, oocysts counts (log10/g), gut lesions, and incidences and the relative microbial abundances and diversity were analyzed as a randomized complete block design (RCBD) by the ANOVAs using the MIXED procedure followed by Tukey's multiple comparison test of SAS 9.4 (SAS Institute Inc., Cary NC). Pens (as replicates) were included as the block and incidence of gut lesions were analyzed using Proc Freq of SAS. Only taxa with >0.05% mean relative abundance in at least one treatment group were included in the analysis. Block, vaccination, treatments, and the interaction between vaccination and treatments were considered as fixed effects. The results were expressed as the least square means and standard error of the mean (SEM). A P value < 0.05 was used to declare significance.
Results
Growth Performances
In general, vaccination significantly decreased (P < 0.05) BW on days 20 and 28, while BAC, CIN, CIT, and CIT + CIN significantly increased (P < 0.05) BW compared with the control on day 28. At day 10, only BAC increased (P < 0.05) the BW compared with the control. The interactions (P < 0.05) between vaccination and treatments (control, BAC, CIN, CIT, and CIN + CIT) on affecting BW were noted on day 10. For ADFI, no differences were observed between vaccinated and nonvaccinated broilers in the starter, grower, finisher, and the whole phase (day 0–28). Similarly, the broilers fed CIN, CIT, and CIN + CIT showed ADFI that is similar to the control and BAC. In this study, birds vaccinated against coccidiosis showed a decreased (P < 0.05) ADG in the whole phase. During the grower, a significant effect on ADG was noted only with BAC and CIN + CIT (P < 0.05). Vaccination was found to reduce (P < 0.05) FCR only in the starter, grower, and whole phase. The CIN, CIT, and CIN + CIT showed the effects similar to the BAC treatment group, whereby there was a significant reduction (P < 0.05) of FCR in the grower, finisher, and whole phase. Like BAC, tested encapsulated oils (either alone or in combination) significantly reduced mortality rates compared to the control (P < 0.01, Table 2).
Table 2.
Effect of bacitracin, encapsulated cinnamaldehyde and citral on growth performance of broilers vaccinated (PV) or nonvaccinated (PNV).
| Phases | Items1 | Vaccination2 |
Treatments3 |
SEM | Effects4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNV | PV | Control | BAC | CIN | CIT | CIN + CIT | Vac | Trt | Vac × Trt | |||
| Starter, 0–9 D | BW (0 D, g) | 45 | 45 | 45 | 45 | 45 | 45 | 45 | - | - | - | - |
| ADFI, g/D | 30 | 30 | 29 | 30 | 29 | 30 | 30 | 0.452 | ns | ns | ns | |
| ADG, g/D | 24 | 24 | 23 | 25 | 23 | 24 | 24 | 0.639 | ns | ns | * | |
| FCR, g/g | 1.23b | 1.26a | 1.26a | 1.21b | 1.26a,b | 1.26a | 1.25a,b | 0.019 | * | * | ns | |
| Grower, 10–19 D | BW (10 D, g) | 282 | 286 | 280b | 293a | 281b | 283b | 283b | 4.071 | ns | * | * |
| ADFI, g/D | 99 | 100 | 102 | 104 | 94 | 95 | 101 | 3.369 | ns | ns | ns | |
| ADG, g/D | 67 | 64 | 64b | 67a | 66a,b | 63b | 67a | 1.591 | ns | * | ns | |
| FCR, g/g | 1.58a | 1.48b | 1.60a | 1.55a,b | 1.43c | 1.53b | 1.52b | 0.054 | * | * | ns | |
| Finisher, 20–28 D | BW (20 D, g) | 960a | 928b | 931b,c | 971a | 947b | 914c | 958b | 18.954 | * | * | ns |
| ADFI, g/D | 158 | 157 | 150 | 162 | 159 | 160 | 157 | 3.499 | ns | ns | ns | |
| ADG, g/D | 94 | 90 | 80b | 102a | 95a,b | 94a,b | 90a,b | 6.197 | ns | * | ns | |
| FCR, g/g | 1.754 | 1.723 | 1.94a | 1.59c | 1.69b | 1.71b | 1.76b | 0.097 | ns | * | ns | |
| Whole phase, 0–28 D | BW (28 D, g) | 1,727a | 1,662b | 1,593c | 1,787a | 1,732a,b | 1,680b | 1,683b | 56.97 | * | * | ns |
| ADFI, g/D | 99 | 97 | 102 | 98 | 97 | 96 | 96 | 1.959 | ns | ns | ns | |
| ADG, g/D | 63a | 60b | 60b | 64a | 63a,b | 61b | 60b | 1.591 | * | * | ns | |
| FCR, g/g | 1.61a | 1.57b | 1.69a | 1.54c | 1.54b,c | 1.58b | 1.60b | 0.049 | * | * | ns | |
| Mortality, % | 9.31a | 6.88b | 15.16a | 3.61d | 10.31b | 5.31c | 6.09c | 18.702 | * | ** | ns | |
a,b,c,dMeans in rows with different letters differ (P < 0.05).
BW, body weight; ADFI, average daily feed intake; ADG, average daily gain; FCR, feed conversion ratio.
PV, birds vaccinated with a live coccidiosis vaccine; PNV, birds not vaccinated with vaccine.
Control, basal diet; BAC, 55 ppm bacitracin; CIN, 100 ppm encapsulated cinnamaldehyde; CIT, 100 ppm encapsulated citral; CIN + CIT, a combination of 100 ppm encapsulated cinnamaldehyde and citral.
Vac, main effect of vaccination; Trt, main effects of treatments; Vac × Trt, interaction between vaccination and treatments. Asterisks indicate significant statistically differences (1 asterisk means a significance level of 0.05 and 2 asterisks 0.01).
General and Intestinal Health
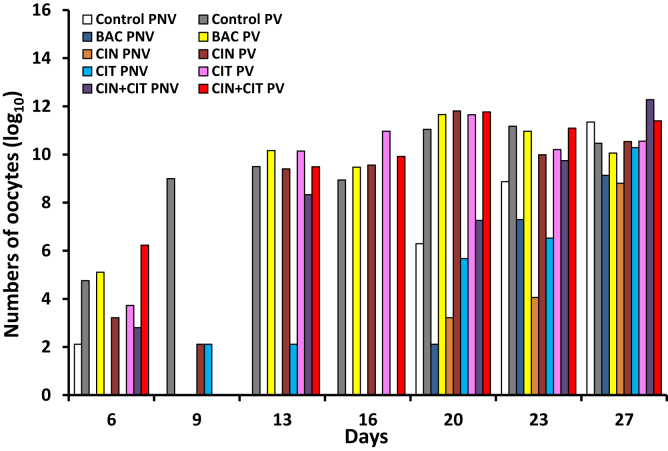
No treatment effects were noted on the oocyst counts in fecal materials collected from 6 to 27 D (Figure 1). As expected, birds that received vaccine showed a significant highest oocyst counts compared with birds that did not receive this vaccine (P < 0.01). In nonvaccinated birds, the highest oocyst counts were observed on day 27, while increased oocyst counts were observed in vaccinated birds from days 6 to 27. On day 27, the nonvaccinated group showed the highest oocyst counts in the control and CIN + CIT fed birds (P < 0.01).
Figure 1.
Effects of vaccination and encapsulated cinnamaldehyde and citral in diets of broilers on oocysts counts in fecal samples at days 6, 9, 13, 16, 20, 23, and 27 of age. Birds vaccinated (PV) and not vaccinated (PNV) against coccidiosis; Control, birds fed basal diet; BAC, birds fed basal diet with 55 ppm bacitracin; CIN, birds fed basal diet with 100 ppm encapsulated cinnamaldehyde; CIT, birds fed basal diet with 100 ppm encapsulated citral; CIN + CIT, birds fed basal diet with 100 ppm encapsulated cinnamaldehyde and 100 ppm citral. Data were affected by vaccination (P < 0.05) but it was unaffected by BAC, CIN, CIT, and CIN + CIT.
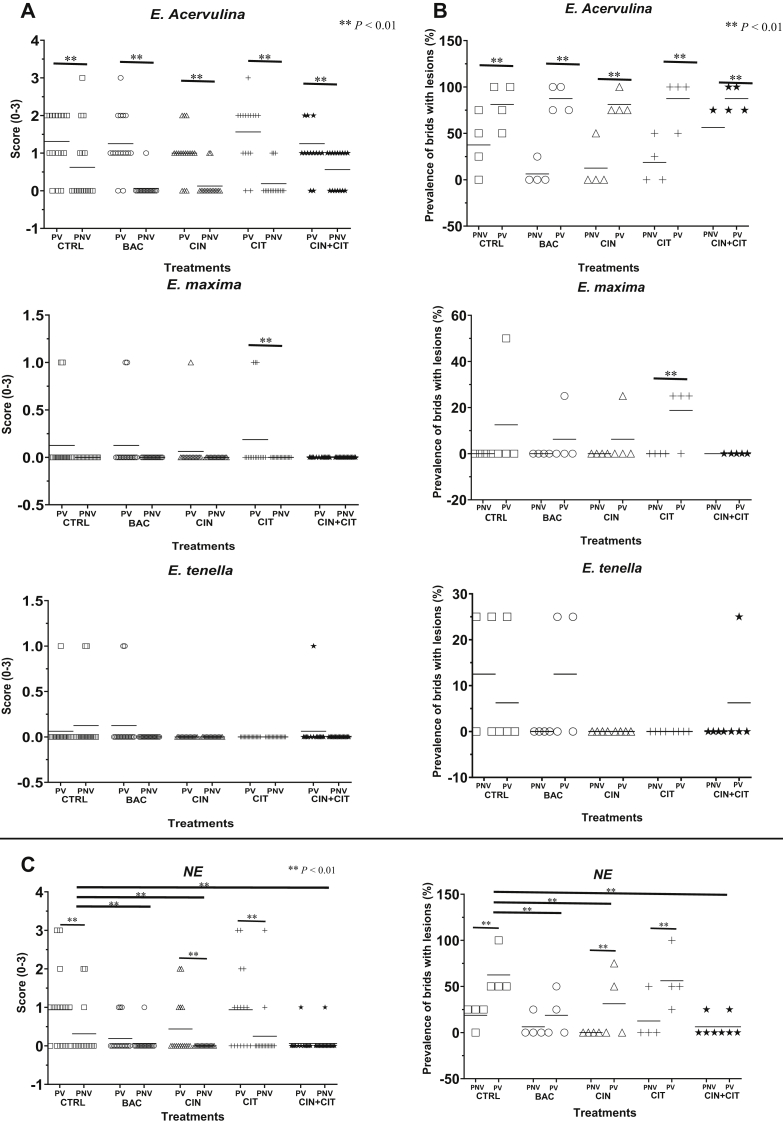
Coccidiosis: In general, subclinical (minor low lesion scores) coccidiosis which was more prevalent in vaccinated than nonvaccinated birds, were observed (Figures 2A and 2B).
Figure 2.
Effects of bacitracin and encapsulated cinnamaldehyde and citral in diets of broilers on (A) severity and (B) prevalence of coccidiosis lesions due to E. acervuline, E. maxima, E. tenella; necrotic enteritis lesions on (C) severity and (D) prevalence due to C. perfringens in both vaccinated (PV) or nonvaccinated (PNV) chickens at 21–22 D of age. CTRL, birds fed basal diet; BAC, birds fed basal diet with 55 ppm bacitracin; CIN, birds fed basal diet with 100 ppm encapsulated cinnamaldehyde; CIT, birds fed basal diet with 100 ppm encapsulated citral; CIN + CIT, birds fed basal diet with 100 ppm encapsulated cinnamaldehyde and 100 ppm citral. Data were not affected by the Vac × Trt (P > 0.10).
In the nonvaccinated group, coccidiosis lesions due to E. acervulina were observed in the control, BAC-, CIN-, CIT-, and CIN + CIT-treated birds with an incidence being 37.5%, 6.3%, 6.3%, 18.8%, and 56.3%, respectively. Birds with a lesion core of 3 (numerous coalescent lesions in the duodenum) were found only in the control. Data suggested that encapsulated CIN at 100 ppm in feed could provide the results similar to BAC at 55 ppm in controlling coccidiosis due to E. acervulina in nonvaccinated broiler chicken.
In vaccinated birds, E. acervulina was the most prevalent Eimeria ssp examined (regardless of treatments) with more than 80% of birds in each treatment group showing intestinal lesions due to this parasite. However, the average lesion scores due to E. acervulina were 1.3, 1.2, 1.0, 1.6, and 1.3 for the control, BAC-, CIN-, CIT- and CIN + CIT-treated groups, respectively. Low incidence and minor coccidiosis lesion scores by E. maxima (6.3 to 18.8% of birds with average lesion scores of 0.2 to 0.1) and E. tenella (6.3 to 12.5% of birds with average lesion scores of 0.1) were observed. No lesions due to E. maxima were found in CIN + CIT-treated birds and no lesions due to E. tenella were observed in each of CIN- and CIT-treated birds.
Necrotic enteritis: Significant effects of the tested EOs were observed on reducing NE incidence and severity, which were comparable to the effects of bacitracin (Figure 2C).
The nonvaccinated control birds showed a 19% incidence of NE with an average lesion score being 0.2 of severity (the maximum severity was 3 found mainly in the control and CIT-fed birds). In general, the CIT-treated birds showed a similar level of NE incidence and severity as the control birds, while birds treated with CIN showed 0% NE incidence and severity. An incidence of 6.3% and an average severity lesion score of 0.1 of NE were observed in the bacitracin-treated group. The CIN + CIT-treated birds resulted in a similar level NE incidence and severity as the BAC-treated group.
Among the birds vaccinated against coccidiosis, the control birds (no supplemented feed) showed an NE prevalence of 63% with an average lesion severity score of 1 (occasional lesion consisting of small areas of erosion, necrosis, or hemorrhage). Nineteen percent of birds (average lesion score = 0.2) of the BAC-treated birds presented NE while 56% (average lesion scores = 0.9) and 31% (average lesion scores = 0.3) of CIT- and CIN-treated birds showed NE lesion scores. The CIN + CIT-treated birds resulted in 6% incidence and 0.1 average severity of NE, which suggested a better control of NE disease than BAC (19% and lesion score of 0.9). It appeared that encapsulated CIN at 100 ppm was more active than CIT at the same dose in controlling NE, although an additive effect appeared to exist when the 2 oils were combined. Overall, the data indicated that the encapsulated essential CIN at the test dose and its combination with CIT could be used as an alternative to dietary bacitracin to combat NE disease in broiler production.
Cecal Microbiota
Four bacterial phyla (Firmicutes, Proteobacteria, Tenericutes, and Actinobacteria) were detected by 16S rRNA gene sequencing analysis, with Firmicutes (90%) being the predominant phylum. Vaccination and treatments alone or their interaction did not affect the relative abundance of phyla (Table 3). At the genus level, a significant reduction in the relative abundance of Lactobacillus (P < 0.05) in the vaccinated group was observed. Ceca from broilers received feed supplemented with CIT, showed an increased relative abundance of Lactobacillus (P < 0.01) but a decreased Ruminococcus (P < 0.05) compared with the control and BAC-fed birds. In addition, feed supplemented with CIN, CIT, and CIN + CIT reduced relative abundances of Enterococcus (P < 0.05) and Clostridium (P < 0.01) in ceca of birds compared with control feed which is similar to BAC. In addition, Clostridium was reduced (P < 0.05) in the vaccinated group with interactions between vaccination and treatments being found. However, interactions between vaccination and treatments were observed for the relative abundances of Ruminococcus (P < 0.05), and Faecalibacterium (P < 0.01), but not for, Oscillospira and Dorea (Table 3).
Table 3.
Relative abundance of phyla and major genera (each representing >1.0% of total sequences on average) in ceca.
| Phyla/Genera | Vaccination1 | Treatments2 |
SEM | Effects3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | BAC | CIN | CIT | CIT + CIN | Vac | Trt | Vac × Trt | |||
| p_Actinobacteria | PV | 0.02 | 0.01 | 0.01 | 0.03 | 0.04 | 0.019 | ns | ns | ns |
| PNV | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 | |||||
| p_Firmicutes | PV | 97.44 | 94.16 | 96.71 | 96.76 | 94.98 | 0.485 | ns | ns | ns |
| PNV | 96.88 | 96.26 | 92.62 | 93.71 | 95.13 | |||||
| p_Proteobacteria | PV | 1.44 | 2.89 | 2.23 | 2.28 | 4.53 | 4.460 | ns | ns | ns |
| PNV | 2.14 | 2.84 | 6.51 | 5.94 | 3.82 | |||||
| p_Tenericutes | PV | 0.83 | 2.86 | 0.81 | 0.80 | 0.39 | 1.415 | ns | ns | ns |
| PNV | 0.91 | 0.73 | 0.79 | 0.46 | 0.91 | |||||
| p_unassigned | PV | 0.26 | 0.08 | 0.24 | 0.13 | 0.12 | 0.120 | ns | ns | ns |
| PNV | 0.06 | 0.14 | 0.05 | 0.06 | 1.63 | |||||
| g_Lactobacillus | PV | 10.70 | 8.90 | 3.76 | 17.79 | 3.09 | 0.637 | * | ** | ns |
| PNV | 5.82 | 5.80 | 3.57 | 11.11 | 1.74 | |||||
| g_Ruminococcus | PV | 13.05 | 11.60 | 11.10 | 7.42 | 12.33 | 2.230 | ns | * | * |
| PNV | 11.39 | 12.25 | 11.33 | 10.33 | 7.53 | |||||
| g_Oscillospira | PV | 8.87 | 11.53 | 11.02 | 9.62 | 12.80 | 3.114 | ns | ns | ns |
| PNV | 10.18 | 10.71 | 9.08 | 9.96 | 8.06 | |||||
| g_Faecalibacterium | PV | 2.78 | 7.55 | 4.61 | 6.09 | 6.46 | 2.940 | ns | ns | * |
| PNV | 8.62 | 5.36 | 8.11 | 5.25 | 3.18 | |||||
| g_Dorea | PV | 1.26 | 1.27 | 1.14 | 0.69 | 1.40 | 0.498 | ns | ns | ns |
| PNV | 1.35 | 1.42 | 1.29 | 1.56 | 0.87 | |||||
| g_Enterococcus | PV | 1.21 | 0.04 | 0.05 | 0.13 | 0.08 | 0.107 | ns | * | ns |
| PNV | 1.04 | 0.08 | 0.07 | 0.05 | 0.02 | |||||
| g_Clostridium | PV | 1.00 | 0.17 | 0.20 | 0.15 | 0.19 | 0.214 | * | ** | ns |
| PNV | 1.29 | 0.37 | 0.23 | 0.34 | 0.16 | |||||
PV, birds vaccinated with a live coccidiosis vaccine; PNV, birds not vaccinated with vaccine.
BAC, 55 ppm bacitracin; CIN, 100 ppm encapsulated cinnamaldehyde; CIT, 100 ppm encapsulated citral; CIN + CIT, a combination of 100 ppm encapsulated cinnamaldehyde and citral.
Vac, main effect of vaccination; Trt, main effects of treatments; Vac × Trt, interaction between vaccination and treatments. Asterisks indicate significant statistically differences (1 asterisk means a significance level of 0.05 and 2 asterisks 0.01).
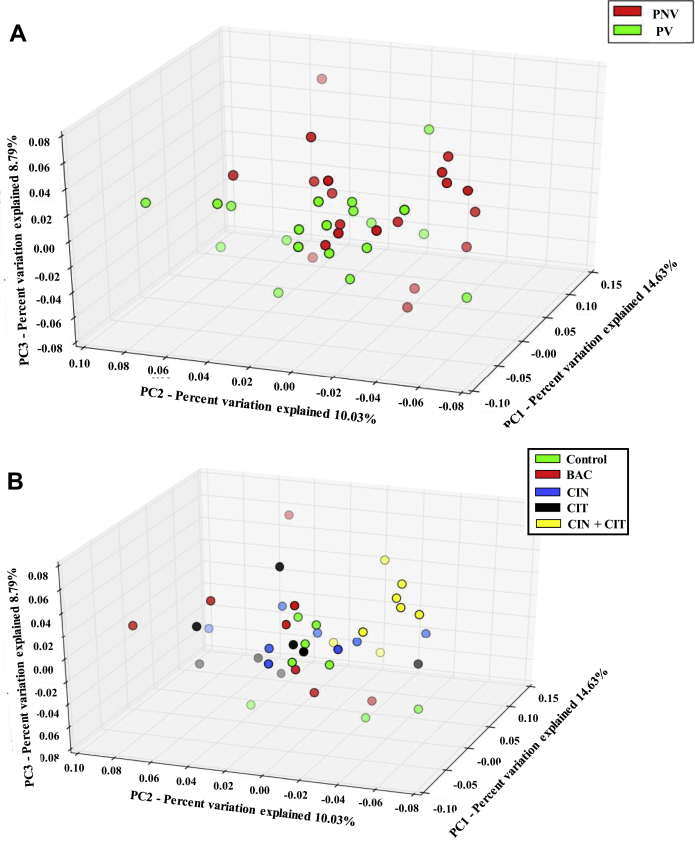
The microbiota richness was estimated using observed OTUs, and the diversity was evaluated by Chao1, Shannon and Simpson indices. Vaccination did not affect microbiota richness and diversity. Feed supplemented with BAC, CIN, CIT alone showed lower (P < 0.05) Simpson compared to the control and BAC supplemented feed (Table 4). The PCoA of the microbiota based on unweighted UniFrac phylogenetic distances followed with PERMANOVA showed that the majority of the samples from the nonvaccinated group (red circle) and vaccinated groups (green circle) clustered separately (Figure 3A); however, no significant differences between dietary treatments in the microbiota composition were observed (Figure 3B).
Table 4.
Summary of alpha-diversity measurements of microbiota in ceca of vaccinated and nonvaccinated broilers treated with bacitracin, cinnamon, citral alone, or in combination.
| Vaccination1 | Treatments2 |
SEM | Effects3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | BAC | CIN | CIT | CIN + CIT | Vac | Trt | Vac × Trt | |||
| Observed OTUs | PV | 393.38 | 396.95 | 409.50 | 389.60 | 383.88 | 18.88 | Ns | ns | ns |
| PNV | 398.67 | 384.78 | 399.75 | 391.20 | 400.10 | |||||
| Chao1 | PV | 431.91 | 436.71 | 444.70 | 427.54 | 419.26 | 15.72 | Ns | ns | ns |
| PNV | 437.41 | 421.36 | 436.50 | 428.08 | 438.48 | |||||
| Shannon | PV | 6.47 | 6.34 | 6.67 | 5.85 | 6.60 | 0.36 | Ns | ns | ns |
| PNV | 6.54 | 6.42 | 6.42 | 6.22 | 6.61 | |||||
| Simpson | PV | 0.97 | 0.97 | 0.98 | 0.94 | 0.98 | 0.016 | Ns | * | ns |
| PNV | 0.98 | 0.97 | 0.97 | 0.96 | 0.98 | |||||
PV, birds vaccinated with a live coccidiosis vaccine; PNV, birds not vaccinated with vaccine.
BAC, 55 ppm bacitracin; CIN, 100 ppm encapsulated cinnamaldehyde; CIT, 100 ppm encapsulated citral; CIN + CIT, a combination of 100 ppm encapsulated cinnamaldehyde and citral.
Vac, main effect of vaccination; Trt, main effects of treatments; Vac × Trt, interaction between vaccination and treatments. Asterisks indicate significant statistically differences (1 asterisk means a significance level of 0.05).
Figure 3.
The 3D principal coordinate analysis (PCoA) graph shows the variation among distance matrixes (unweighted UniFrac) of cecal microbiota in (A) vaccinated or nonvaccinated status and (B) treatments with bacitracin, encapsulated cinnamaldhyde and citral, alone or in the combination. Percentages shown are percentages of variation explained by the PC1 (14.64%), PC2 (10.03%), and PC3 (8.79%). PV, birds were vaccinated against coccidiosis; PNV, birds were not vaccinated against coccidiosis; Control, birds fed basal diet; BAC, birds fed basal diet with 55 ppm bacitracin; CIN, birds fed basal diet with 100 ppm encapsulated cinnamaldehyde; CIT, birds fed basal diet with 100 ppm encapsulated citral; CIN + CIT, birds fed basal diet with 100 ppm encapsulated cinnamaldehyde and 100 ppm citral.
Discussion
Since the restrictions on antibiotic use in feed-additives, the search for affordable alternatives has been a growing discipline (Manges et al., 2007). In this study, vaccine and encapsulated CIN and CIT were used alone and in combination as alternatives for preventing naturally incidences of coccidiosis and NE in broilers. Compared with previous studies (Aguilar et al., 2013, Khattak et al., 2013), the EOs in this research were fed at the beginning of the grower (day 10–28) instead of the starter. This is based on our previously reported experimental infection studies, in which EOs were applied from day 10 to day 28 and NE was reduced to the level similar to the treatment with antibiotics in the feed (Liu et al., 2016, Yang et al., 2016). In addition, we speculated that feeding EOs to broilers from the grower instead of starter could receive higher efficacy to promote the growth of broilers because the gut microbiota is not established and stable in the starter (Lu et al., 2003, Gong et al., 2008). As the cecum harbors more diverse and stable microbial communities than the ileum in broilers (Gong et al., 2002, Gong et al., 2007), we selected cecal digesta for analyzing microbiota in the present study.
The results suggested that vaccinations and EOs have interactions on increasing ADG in starter broilers and BW in grower broilers, respectively. This could be explained by the relationship between coccidiosis and NE, which reports that coccidiosis due to Eimeria could enhance mucus production and release plasma proteins by damaging gut epithelial cells, increasing nutrient availability for C. perfringens to grow (Williams et al., 2003). The results indicated that the combination of vaccines and EOs may improve the performance of broilers by controlling pathogens including Eimeria and C. perfringens. In the whole phase, the broilers supplemented with CIN, CIT, and CIN + CIT had similar effects as the BAC treatment, whereby BW was increased and FCR was decreased compared with the control. A previous study has shown that the higher nutrient digestibility associated with growth performance was due to increased secretions of endogenous digestive enzymes stimulated by EOs in the whole phase (Lee et al., 2003). Whether this is applied to the observation reported in the present study remains to be determined. Studies regarding the synergistic effects of EOs containing terpenes on growth performance have been conducted but no studies have examined synergism in an aldehyde and terpene blend, which were the compounds of CIN and CIT, respectively (Siani et al., 2013). In the present study, however, there were no significant synergistic effects of CIN and CIT on growth performance compared with CIN or CIT alone on growth performance. In addition, there were no observed differences among treatment groups regarding ADFI, suggesting that chickens may not sense the flavor of EOs because of their encapsulation. This finding is consistent with previously published studies reporting that feed intake was not significantly reduced by dietary inclusion of EOs (Brenes and Roura, 2010, Bozkurt et al., 2014). This study also indicated that CIN, CIT, and CIN + CIT could reduce mortality, similar to the BAC treatment. In contrast, the mortality in the control was 15%. It could be associated with the environment, management, and diet during the animal trial. For example, high dietary protein content in this study (25.30, 23.30, and 21.30% CP in the starter, grower, and finisher, respectively) may have increased morality, as reported previously in both broilers (Gibson et al., 1989) and laying hens (Pearson and Herron, 1982).
Coccidiosis is a major parasitic infection in broilers, which increases mortality (Wealleans et al., 2017). In this study, the mortality rate was reduced after administering the oocyst vaccine, indicating its effectiveness in controlling coccidiosis. The lower BW and ADG in vaccinated broilers may have been due to a cell-mediated immune response to the oocyst vaccine that contains Eimeria species (Johnson, 1997). It has been reported that the immune response could affect the growth performance of chickens, resulting in reduced ADG (Ahmad et al., 2016). In addition, the previous research also found that the coccidial vaccination may depress the growth of broilers as birds may not able to protect themselves against Eimeria infections (Williams, 2002). The lower FCR in the grower and whole phase indicated that vaccination could increase feed efficiency. The results were in accordance with a previous study indicating that the Eimeria challenge induced by vaccination could improve the growth performance of broilers by lowering the FCR (Lee et al., 2011). However, higher FCR in starter suggested that vaccination may reduce FCR in young birds (Yang et al., 2011).
The minor coccidiosis incidences and lesions due to E. maxima and E. tenella may be due to the high hygienic and biosecurity practice in this study (Diarra et al., 2007). However, coccidiosis due to E. acervulina and NE incidences increased after vaccination. The appearance of coccidiosis lesions could be explained that the immune response to the live coccidiosis vaccine could repeat reinfection through the ingestion of sporulated oocysts sprayed over the surface of feed (Reid, 1990). In addition, it has been reported that factors including Eimeria infection and dietary fish meal could be responsible for inducing NE lesions (Stanley et al., 2014, Wu et al., 2014). In this study, the increased NE incidences in vaccinated birds could be at least partially due to the appearance of Eimeria infection (coccidiosis lesions) and fish meal applied in the diet.
Many EOs including CIN and CIT have been studied in vitro for their potential to inhibit pathogens that cause diseases in chickens (Friedman et al., 2002, Friedman et al., 2004, Si et al., 2009, Giteru et al., 2015). The reduction in coccidiosis incidences and lesions caused by E. acervulina after feeding CIN at 150 ppm has been demonstrated by a previous study (Orengo et al., 2012). However, in the present study, the coccidiosis incidence and lesions due to E. acervulina decreased in birds fed 100 ppm CIN. The results indicated that the protection of the EOs through encapsulation could result in lowering the concentration of CIN to decrease coccidiosis incidence and severity. Accordingly, the results in this study also indicated that both CIT and CIN supplementation possess the ability to reduce coccidiosis. As coccidiosis has been reported to promote NE (Williams et al., 2003), the decreased NE incidence in the present study could be due to the reduction of coccidiosis by CIN and CIN + CIT. Besides, CIN (aldehyde), and CIT (terpene) have been reported to act synergistically (Caldas et al., 2015). To fully understand the molecular mechanisms underlying the effects of CIN and CIT individually or in combination in promoting chicken gut health, more studies are required.
Oocyst counts in feces from broilers are the reflection of coccidial infection in the birds (Hodgson, 1970). The higher fecal oocyst counts in vaccinated birds was consistent with the results of coccidiosis lesions.
The cecal microbiota of broilers can reflect feed digestion and nutrient absorption (Rinttilä and Apajalahti, 2013), which is related to urine recycling and gut health (Karasawa, 1999). In the present study, 4 phyla of microbiota were detected, with Firmicutes (90%) being the predominant phylum, followed by Proteobacteria, Tenericutes, and Actinobaceria. The results were in agreement with previous studies on broilers (Sakaridis et al., 2018, Biasato et al., 2019), although a higher relative abundance of phylum Firmicutes was observed. No Bacteroidetes were detected in the present study, which was also reported by others previously (Han et al., 2016, Pedroso et al., 2016, Lucke et al., 2018).
At the genus level, Lactobacillus, Ruminococcus, Faecalibacterium, Enterococcus, and Clostridium showed significant differences in relative abundance between the control and treatment groups in the present study. Previously Lactobacillus (L. aviarius in particular) has been reported to negatively correlate with the abundance of C. perfringens in broilers with NE disease (Feng et al., 2010). In addition, the reduction of Lactobacillus johnsonii by NE (Stanley et al., 2012) and independently by fish meal (Wu et al., 2014) has also been described. Stanley et al. (2012) observed a significant reduction of Wissella confuse in C. perfringens challenged birds of Weissella confusa, a heterofermentative lactic acid-producing bacterium that was previously classified as a member of Lactobacillus but later relocated to Leuconostoc (Collins et al., 1993, Bjorkroth et al., 2002). Antonissen et al. (2016) reported a shift in species composition of Lactobacillus and reduction in butyrate-producing strains that belong to Ruminococcceae family in broilers treated with Eimeria and fish meal. A decrease of Faecalibacterium and Oscillospira in ceca were also detected in broilers with NE disease (Stanley et al., 2012, Lacey et al., 2018). In the present study, the CIT or vaccine treatment alone increased Lactobacillus abundance compared with control and bacitracin treatments, which could suggest a potential benefit to chicken gut health according to the reports above. In contrast, the CIT and vaccine treatments decreased Ruminococcus abundance compared with the control. Ruminococcus is responsible for the degradation of cellulolytic fibre in ruminants, but its role in broilers remains to be further clarified (Koike and Kobayashi, 2009, Mondot et al., 2016). Facecalibacterium is a group of bacteria able to produce butyrate that is a source of energy in broilers (Mondot et al., 2016, Bortoluzzi et al., 2017) and benefits animal gut health in general (Bedford and Gong, 2017). In the present study, neither vaccination nor EOs alone changed the abundance of Faecalibacterium but their combination did. The low relative abundance of Enterococcus may imply less potential for chicken infection, as the density of Enterococcus in feces and digesta has been considered to be an indicator of fecal contamination that is associated with infections such as septicemia in poultry (Gilmore, 2002, Boehm and Sassoubre, 2014). Clostridium contains some pathogenic, but largely nonpathogenic species (Num and Useh, 2014). Although it was proposed to be a factor for predicting the potential of infections (Udaondo et al., 2017), whether it can be well-established needs to be determined. The changes in microbiota composition beneficial to chicken gut health in response to EO treatment have also been suggested previously (Cooper et al., 2013, Rehman et al., 2018). To determine the cause-effect relationship, further studies are required.
In conclusion, encapsulated CIN alone or in combination with encapsulated CIT in feed altered cecal microbiota composition and improved the intestinal health and performance of broiler chickens similar to BAC. Further studies are required to determine if the cecal microbiota changes contribute to the improvement of intestinal health and performance.
Acknowledgments
This research was funded by Agriculture and Agri-Food Canada and the Centre de recherche en sciences animales de Deschambault (CRSAD). The authors would like to acknowledge the staff of CRSAD who helped during the chicken trial and Emily Ammeter (summer student at the University of Manitoba) for her assistance in manuscript revision.
Contributor Information
Joshua Gong, Email: joshua.gong@canada.ca.
Moussa S. Diarra, Email: moussa.diraar@canada.ca.
References
- Aguilar C.A.L., Lima K.R.D.S., Manno M.C., Tavares F.B., Souza V.P.D., Neto F., Lisboa D. Effect of copaiba essential oil on broiler chickens' performance. Acta Sci. Anim. Sci. 2013;35:145–151. [Google Scholar]
- Agyare C., Boamah V.E., Zumbi C.N., Osei F.B. Antimicrobial Resistance – a Global Threa. 2018. Antibiotic use in poultry production and its effects on bacterial resistance. [DOI] [Google Scholar]
- Ahmad T.A., El-Sayed B.A., El-Sayed L.H. Development of immunization trials against Eimeria spp. Trials Vaccinol. 2016;5:38–47. [Google Scholar]
- Antonissen G., Eeckhaut V., Van Driessche K., Onrust L., Haesebrouck F., Ducatelle R., Moore R.J., Van Immerseel F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016;45:308–312. doi: 10.1080/03079457.2016.1152625. [DOI] [PubMed] [Google Scholar]
- Aronesty E. Expression Analysis; Durham, NC: 2011. Ea-utils: Command-Line Tools for Processing Biological Sequencing Data. [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils - a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bedford A., Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2017;4:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasato I., Ferrocino I., Grego E., Dabbou S., Gai F., Gasco L., Cocolin L., Capucchino M.T., Schiavone A. Gut microbiota and mucin composition in female broiler chickens fed diets including Yellow Mealworm (Tenebrio molitor, L.) Animals (Basel) 2019;9:213. doi: 10.3390/ani9050213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkroth K.J., Schillinger U., Geisen R., Weiss N., Hoste B., Holzapfel W.H., Korkeala H.J., Vandamme P. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int. J. Syst. Evol. Microbiol. 2002;52:141–148. doi: 10.1099/00207713-52-1-141. [DOI] [PubMed] [Google Scholar]
- Boehm A.B., Sassoubre L.M. Enterococci as indicators of environmental fecal contamination. In: Gilmore M.S., Clewell D.B., Ike Y., editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet] Massachusetts Eye and Ear Infirmary; Boston, MA: 2014. [Google Scholar]
- Bortoluzzi C., Pedroso A.A., Mallo J.J., Puyalto M., Kim W.K., Applegate T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 2017;96:3981–3993. doi: 10.3382/ps/pex218. [DOI] [PubMed] [Google Scholar]
- Bozkurt M., Aysul N., Küçükyilmaz K., Aypak S., Ege G., Catli A.U., Akşit H., Çöven F., Seyrek K., Çınar M. Efficacy of in-feed preparations of an anticoccidial, multienzyme, prebiotic, probiotic, and herbal essential oil mixture in healthy and Eimeria spp.-infected broilers. Poult. Sci. 2014;93:389–399. doi: 10.3382/ps.2013-03368. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim. Feed Sci. Technol. 2010;158:1–14. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Caldas G.F.R., da Silva OliveiraAraújo A.R., Lafayette A.V., Albuquerque S.S.L., da Costa Silva-Neto G.S., da Costa Silva-Neto J., Costa-Silva J.H., Ferreira F., da Costa J.G.M., Wanderley A.G. Gastroprotective mechanisms of the monoterpene 1, 8-cineole (eucalyptol) PLos. One. 2015;10:e0134558. doi: 10.1371/journal.pone.0134558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell M., Friis C., Marco E., McMullin P., Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- Chicken Farmers of Canada 2017. https://www.chickenfarmers.ca/antibiotics Accessed April 2017.
- Collins M.D., Samelis J., Metaxopoulos J., Wallbanks S. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993;75:595–603. doi: 10.1111/j.1365-2672.1993.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Collier C.T., Van der Klis J.D., Deplancke B., Anderson D.B., Gaskins H.R. Effects of tylosin on bacterial mucolysis, Clostridium perfringens colonization, and intestinal barrier function in a chick model of necrotic enteritis. Antimicrob. Agents Chemother. 2003;47:3311–3317. doi: 10.1128/AAC.47.10.3311-3317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G., Uzal F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 2013;25:314–327. doi: 10.1177/1040638713483468. [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Wilkie D.C., Van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:60–88. [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra M.S., Silversides F.G., Diarrassouba F., Pritchard J., Masson L., Brousseau R., Bonnet C., Delaquis P., Bach S., Skura B.J., Topp E. Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and Enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia coli isolates. Appl. Environ. Microbiol. 2007;73:6566–6576. doi: 10.1128/AEM.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Feng Y., Gong J., Yu H., Jin Y., Zhu J., Han Y. Identification of changes in the composition of ileal bacterial microbiota of broiler chickens infected with Clostridium perfringens. Vet. Microbiol. 2010;140:116–121. doi: 10.1016/j.vetmic.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Friedman M., Henika P.R., Mandrell R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002;65:1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- Friedman M., Henika P.R., Levin C.E., Mandrell R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157: H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 2004;52:6042–6048. doi: 10.1021/jf0495340. [DOI] [PubMed] [Google Scholar]
- Gibson R.M., Bailey C.A., Kubena L.F., Huff W.E., Harvey W.E. Ochratoxin A and dietary protein. 1. Effects on body weight, feed conversion, relative organ weight, and mortality in three-week-old broilers. Poult. Sci. 1989;68:1658–1663. doi: 10.3382/ps.0681658. [DOI] [PubMed] [Google Scholar]
- Gilmore M.S. ASM Press; Washington, DC: 2002. The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance. [Google Scholar]
- Giteru S.G., Coorey R., Bertolatti D., Watkin E., Johnson S., Fang Z. Physicochemical and antimicrobial properties of citral and quercetin incorporated kafirin-based bioactive films. Food Chem. 2015;168:341–347. doi: 10.1016/j.foodchem.2014.07.077. [DOI] [PubMed] [Google Scholar]
- Gong J., Forster R.J., Yu H., Chambers J.R., Wheatcroft R., Sabour P.M., Chen S. Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol. Ecol. 2002;41:171–179. doi: 10.1111/j.1574-6941.2002.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Gong J., Si W., Forster R.J., Huang R., Yu H., Yin Y., Yang C., Han Y. 16S rDNA-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol. Ecol. 2007;59:147–157. doi: 10.1111/j.1574-6941.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- Gong J., Yu H., Liu T., Gill J.J., Chambers J.R., Wheatcroft R., Sabour P.M. Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens. J. Appl. Microbiol. 2008;104:1372–1382. doi: 10.1111/j.1365-2672.2007.03699.x. [DOI] [PubMed] [Google Scholar]
- Griggs J.P., Jacob J.P. Alternatives to antibiotics for organic poultry production. J. Appl. Poult. Res. 2005;14:750–756. [Google Scholar]
- Han Z., Willer T., Pielsticker C., Gerzova L., Rychlik I., Rautenschlein S. Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathog. 2016;8:56. doi: 10.1186/s13099-016-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J.N. Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 1970;28:99–102. doi: 10.1016/0014-4894(70)90073-1. [DOI] [PubMed] [Google Scholar]
- Johnson R.W. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 1997;75:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- Karasawa Y. Significant role of the nitrogen recycling system through the ceca occurs in protein-depleted chickens. J. Exp. Zool. 1999;283:418–425. doi: 10.1002/(sici)1097-010x(19990301/01)283:4/5<418::aid-jez11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Rubbo A.D., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak F., Ronchi A., Castelli P., Sparks N. Aguilar blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult. Sci. 2013;93:132–137. doi: 10.3382/ps.2013-03387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Doi E., Iwata I., Nishimura H. Degradation products of citral by acid: minor components responsible for off-odor of deteriorated lemon. Bull. Chem. Soc. Jpn. 1981;55:1073–1079. [Google Scholar]
- Klindworth A., Pruesse E., Scheweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR sprimers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Kobayashi Y. Fibrolytic rumen bacteria: their ecology and functions. Asian-Australas. J. Anim. Sci. 2009;22:131–138. [Google Scholar]
- Lacey J.A., Stanley D., Keyburn A.L., Ford M., Chen H., Johanesen P., Lyras D., Moore R.J. Clostridium perfringens-mediated necrotic enteritis is not influenced by the pre-existing microbiota but is promoted by large changes in the post-challenge microbiota. Vet. Microbiol. 2018;227:119–126. doi: 10.1016/j.vetmic.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Everts H., Kappert H.J., Frehner M., Losa R., Beynen A.C. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Lee J.T., Eckert N.H., Ameiss K.A., Stevens S.M., Anderson P.N., Anderson S.M., Barri A., McElroy A.P., Danforth H.D., Caldwell D.J. The effect of dietary protein level on performance characteristics of coccidiosis vaccinated and nonvaccinated broilers following mixed-species Eimeria challenge. Poult. Sci. 2011;90:1916–1925. doi: 10.3382/ps.2011-01362. [DOI] [PubMed] [Google Scholar]
- Lepp D., Gong J., Songer J.G., Boerlin P., Parreira V.R., Prescott J.F. Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the netB plasmid. J. Bacteriol. 2013;195:1152–1166. doi: 10.1128/JB.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H.S., Lillehoj E.P. Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis. 2000;44:408–425. [PubMed] [Google Scholar]
- Liu X., Diarra M.S., Zhang Y., Wang Q., Yu H., Nie S.P., Xie M.Y., Gong J. Effect of encapsulated carvacrol on the incidence of necrotic enteritis in broiler chickens. Avian Pathol. 2016;45:357–364. doi: 10.1080/03079457.2016.1138281. [DOI] [PubMed] [Google Scholar]
- Long P., Jeffers T. Control of chicken coccidiosis. Parasitol. Today. 1986;2:236–240. doi: 10.1016/0169-4758(86)90002-5. [DOI] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke A., Böhm J., Zebeli Q., Metzler-Zebeli B.U. Dietary Deoxynivalenol contamination and oral lipopolysaccharide challenge alters the cecal microbiota of broiler chickens. Front. Microbiol. 2018;9:804. doi: 10.3389/fmicb.2018.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.-H., Wang Q., Gong J., Wu X.Y. Formulation of granules for site-specific delivery of an antimicrobial essential oil to the animal intestinal tract. J. Pharm. Sci. 2016;105:1124–1133. doi: 10.1016/j.xphs.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Manges A.R., Smith S.P., Lau B.J., Nuval C.J., Eisenberg J.N., Dietrich P.S., Riley L.W. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: a case–control study. Foodborne Pathog. Dis. 2007;4:419–431. doi: 10.1089/fpd.2007.0026. [DOI] [PubMed] [Google Scholar]
- Martin A.G., Danforth H.D., Barta J.R., Fernando M.A. Analysis of immunological cross-protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of Eimeria maxima. Int. J. Parasitol. 1997;27:527–533. doi: 10.1016/s0020-7519(97)00027-1. [DOI] [PubMed] [Google Scholar]
- McDevitt R.M., D Brooker J., Acamovic T., Sparks N.H.C. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds Poult. Sci. J. 2006;62:221–247. [Google Scholar]
- McDonel J.L. Clostridium perfringens toxins (type a, b, c, d, e) Pharmacol. Ther. 1980;10:617–655. doi: 10.1016/0163-7258(80)90031-5. [DOI] [PubMed] [Google Scholar]
- Mondot S., Lepage P., Seksik P., Allez M., Tréton X., Bouhnik Y., Colombel J.F., Leclerc M., Pochart P., Doré J., Marteau P. Structural robustness of the gut mucosal microbiota is associated with Crohn's disease remission after surgery. Gut. 2016;65:954–962. doi: 10.1136/gutjnl-2015-309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S., Di Lorenzo A., Izadi M., Sobarzo-Sánchez E., Daglia M. Antibacterial effects of cinnamon: from farm to food, cosmetic and pharmaceutical industries. Nutrients. 2015;7:7729–7748. doi: 10.3390/nu7095359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Num S.M., Useh N.M. Clostridium: pathogenic roles, industrial uses and medicinal prospects of natural products as ameliorative agents against pathogenic species. Jordan J. Biol. Sci. 2014;147:1–14. [Google Scholar]
- Ohtsuru M., Teraoka M., Tori K., Takeda K.I. Proton magnetic resonance studies of citral a and b. J. Chem. Soc. 1967;71:1033–1035. [Google Scholar]
- Omonijo F.A., Ni L., Gong J., Wang Q., Lahaye L., Yang C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2017;4:126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onawunmi G.O. Evaluation of the antimicrobial activity of citral. Lett. Appl. Microbiol. 1989;9:105–108. [Google Scholar]
- Orengo J., Buendía A.J., Ruiz-Ibáñez M.R., Madrid J., Del Río L., Catalá-Gregori P., García V., Hernández F. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet. Parasitol. 2012;185:158–163. doi: 10.1016/j.vetpar.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Osman K., Elhariri M. Antibiotic resistance of Clostridium perfringens isolates from broiler chickens in Egypt. Rev. Sci. Tech. 2013;32:841–850. doi: 10.20506/rst.32.2.2212. [DOI] [PubMed] [Google Scholar]
- Pearson R.A., Herron K.M. Effects of maternal energy and protein intakes on the incidence of malformations and malpositions of the embryo and time of death during incubation. Br. Poult. Sci. 1982;23:71–77. doi: 10.1080/00071688208447931. [DOI] [PubMed] [Google Scholar]
- Pedroso A.A., Batal A.B., Lee M.D. Effect of in ovo administration of an adult-derived microbiota on establishment of the intestinal microbiome in chickens. Am. J. Vet. Res. 2016;77:514–526. doi: 10.2460/ajvr.77.5.514. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Parreira V.R., Gohari I.M., Lepp D., Gong J. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know. Avian Pathol. 2016;45:288–294. doi: 10.1080/03079457.2016.1139688. [DOI] [PubMed] [Google Scholar]
- Price M.N., Dehal P.S., Arkin A.P. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman M.A., Yin X., Zaheer R., Goji N., McAllister T., Pritchard J., Topp E., Diarra M.S. Genotypes and phenotypes of Enterococci isolated from broiler chickens. Front. Sustain. Food Syst. 2018;2:83. [Google Scholar]
- Reid W.M. History of avian medicine in the United States. X. Control of coccidiosis. Avian Dis. 1990;34:509–525. [PubMed] [Google Scholar]
- Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites—implications for broiler chicken health and performance1. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Sakaridis I., Ellis R.J., Cawthraw S.A., Van Vliet A.H., Stekel D.J., Penell J., Chambers M., La Ragione R.M., Cook A.J. Investigating the association between the caecal microbiomes of broilers and Campylobacter burden. Front. Microbiol. 2018;9:927. doi: 10.3389/fmicb.2018.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang-Oh P., Chae-Min R., Byung-Sung P., Jong H. The meat quality and growth performance in broiler chickens fed diet with cinnamon powder. J. Environ. Biol. 2013;34:127–133. [PubMed] [Google Scholar]
- Si W., Gong J., Tsao R., Zhou T., Yu H., Poppe C., Johnson R., Du Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006;100:296–305. doi: 10.1111/j.1365-2672.2005.02789.x. [DOI] [PubMed] [Google Scholar]
- Si W., Ni X., Gong J., Yu H., Tsao R., Han Y., Chambers J.R. Antimicrobial activity of essential oils and structurally related synthetic food additives towards Clostridium perfringens. J. Appl. Microbiol. 2009;106:213–220. doi: 10.1111/j.1365-2672.2008.03994.x. [DOI] [PubMed] [Google Scholar]
- Siani A.C., Souza M.C., Henriques M.G., Ramos M.F. Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Biol. 2013;51:881–887. doi: 10.3109/13880209.2013.768675. [DOI] [PubMed] [Google Scholar]
- Silva C.D.B.D., Guterres S.S., Weisheimer V., Schapoval E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008;12:63–66. doi: 10.1590/s1413-86702008000100014. [DOI] [PubMed] [Google Scholar]
- Stanley D., Keyburn A.L., Denman S.E., Moore R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012;159:155–162. doi: 10.1016/j.vetmic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S.B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9:e104739. doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W.L., Lei L.L., Zhang Q., Li Y. Physical stability and antimicrobial activity of encapsulated cinnamaldehyde by self-emulsifying nanoemulsion. J. Food Process Eng. 2016;39:462–471. [Google Scholar]
- Tisserand R., Balacs T. Churchill Livingstone; Edinburgh: 1995. Essential Oil Safety. [Google Scholar]
- Udaondo Z., Duque E., Ramos J.L. The pangenome of the genus C lostridium. Environ. Microbiol. 2017;19:2588–2603. doi: 10.1111/1462-2920.13732. [DOI] [PubMed] [Google Scholar]
- Wallach M. Role of antibody in immunity and control of chicken coccidiosis. Trends Parasitol. 2010;26:382–387. doi: 10.1016/j.pt.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Wang L., Hou Y., Yi D.D., Ding B., Zhao D., Wang Z., Zhu H., Liu Y., Gong J., Assaad H., Wu G. Beneficial roles of dietary oleum cinnamomi in alleviating intestinal injury. Front. Biosci. (Landmark Ed) 2015;20:814–828. doi: 10.2741/4339. [DOI] [PubMed] [Google Scholar]
- Wealleans A.L., Li W., Romero L.F., Mathis G., Lumpkins B. Performance and cost-benefit improvements following supplementation with a combination of direct-fed microbials and enzymes to broiler chickens raised with or without ionophores. J. Appl. Poult. Res. 2017;27:23–32. [Google Scholar]
- Williams R.B. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. 2002;31:317–353. doi: 10.1080/03079450220148988. [DOI] [PubMed] [Google Scholar]
- Williams R.B., Marshall R.N., La Ragione R.M., Catchpole J. A new method for the experimental production of necrotic enteritis and its use for studies on the relationships between necrotic enteritis, coccidiosis and anticoccidial vaccination of chickens. Parasitol. Res. 2003;90:19–26. doi: 10.1007/s00436-002-0803-4. [DOI] [PubMed] [Google Scholar]
- Wu S.B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Yang X.J., Li W.L., Feng Y., Yao J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011;90:2740–2746. doi: 10.3382/ps.2011-01591. [DOI] [PubMed] [Google Scholar]
- Yang C., Chowdhury M.A.K., Hou Y., Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 2015;4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Cui S., Gong J., Miller S.S., Wang Q., Hua Y. Stability of citral in oil-in-water emulsions protected by a soy protein–polysaccharide Maillard reaction product. Food Res. Int. 2015;69:357–363. [Google Scholar]
- Yang Y., Wang Q., Diarra M.S., Yu H., Hua Y., Gong J. Functional assessment of encapsulated citral for controlling necrotic enteritis in broiler chickens. Poult. Sci. 2016;95:780–789. doi: 10.3382/ps/pev375. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gong J., Yu H., Guo Q., Defelice C., Hernandez M., Yin Y., Wang Q. Alginate-whey protein dry powder optimized for target delivery of essential oils to the intestine of chickens. Poult. Sci. 2014;93:1–12. doi: 10.3382/ps.2013-03843. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Q.C., Yu H., Zhu J., de Lange C.F.M., Yin Y., Wang Q., Gong J. Evaluation of alginate-whey protein microcapsules for intestinal delivery of lipophilic compounds in pigs. J. Sci. Food Agric. 2015;96:2674–2681. doi: 10.1002/jsfa.7385. [DOI] [PubMed] [Google Scholar]