Figure 1.
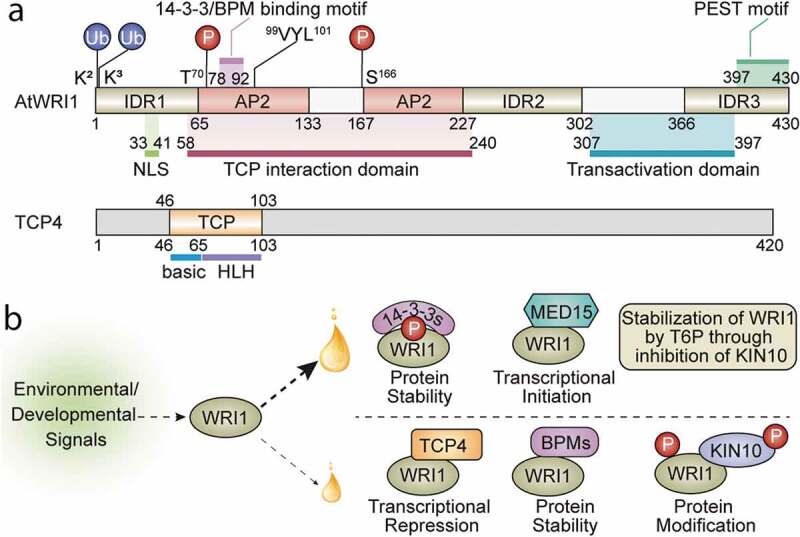
Structural features of WRI1 and TCP4 and molecular mechanisms of WRI1-regulated oil biosynthesis. (a) Schematic diagram of AtWRI1, including two AP2 domains, three intrinsically disordered regions (IDRs), a nuclear localization signal (NLS), a functional motif of “VYL”, the 14-3-3 and E3 ligase adaptor (BPM) binding motifs, TCP4-interacting domain, the transactivation domain (TAD), the ubiquitination sites, and the KIN10 phosphorylation sites. The schematic diagram of TCP4 shows the basic region and the helix-loop-helix (HLH) region of the TCP domain. (b) The fine-tuning of lipid biosynthesis through modulating WRI1 activity leads to an increase (large oil drop) or a decrease (small oil drop) of oil accumulation. In response to environmental or developmental signals, various WRI1 modules are formed, which either positively or negatively regulate the transcriptional activity of WRI1. Interaction of WRI1 with 14-3-3s or MED15 leads to enhanced protein stability, transcriptional activity, or assembly of the transcriptional machinery, resulting in higher oil accumulation. T6P stabilizes WRI1 by inhibition of KIN10, which positively mediates oil biosynthesis. Modules formed between WRI1 and TCP4, BPMs, or KIN10 reduce transcriptional activity or protein stability, resulting in lower oil accumulation. 14-3-3s, 14-3-3 proteins; MED15, Mediator subunit 15; T6P, trehalose 6-phosphate; BPMs, CULLIN3-based E3 ligase adaptor BTB/POZMATH (BPM) proteins
