Abstract
Objective
Ovarian cancer is one of three malignant tumors of the female reproductive system. Our previous studies showed that the traditional Chinese medicine naringin significantly inhibited the proliferation of platinum-resistant ovarian cancer cells in vitro, and that the mechanism may be related to the NF-κB pathway.
Methods
The MTT assay was used to detect the sensitivity of SKOV3 and SKOV3/CDDP cells to cisplatin, the effect of different naringin concentrations on the proliferation of SKOV3/CDDP cells, and the reversal of cisplatin resistance in naringin-treated SKOV3/CDDP cells. Western blotting was used to detect β-catenin, c-Myc, and cyclin D1 protein levels in the different cell lines.
Results
MTT results showed that different concentrations of naringin inhibited the proliferation of SKOV3 and SKOV3/CDDP cells, and that the inhibition increased with increasing concentrations and prolonged incubation times. Western blotting revealed that compared with controls (SKOV3/CDDP-0), β-catenin, c-Myc and cyclin D1 proteins levels were significantly decreased in SKOV3/CDDP-C, SKOV3/CDDP-N 20, and SKOV3/CDDP-CN 20 cells, suggesting that naringin inhibited the proliferation of SKOV3/CDDP cells in a concentration and time dependent manner.
Conclusions
Non-cytotoxic naringin reduced the expression of β-catenin, c-Myc, and cyclin D1 in SKOV3/CDDP cells and partially reversed cisplatin resistance in SKOV3/CDDP CN 20 cells.
Keywords: Ovarian cancer, naringin, SKOV3/CDDP, cisplatin, cisplatin resistance, Wnt/β-catenin signaling pathway
Introduction
Ovarian cancer is the third most common malignant tumor of the female reproductive system, following cervical cancer and uterine cancer. Owing to the lack of specific symptoms and effective screening strategies for early-stage disease, most ovarian cancer cases progress to advanced stages after diagnosis. Ovarian cancer is the leading cause of death from malignancies of the female reproductive system owing to its difficulty to treat, high recurrence rate, and rapid metastasis.1 Effective treatments for ovarian cancer include surgical resection and platinum-based chemotherapy, which have improved the prognosis of patients to some extent.2 However, in clinical treatment, ovarian cancer cells often become cisplatin resistant, leading to treatment failure, which directly affects both tumor-free and long-term survival rates. The postoperative recurrence rate of ovarian cancer can be as high as 85%, and the 5-year survival rate is less than 30%.3,4 Therefore, developing new strategies to reverse cisplatin resistance has become a major research goal for ovarian cancer.
Traditional Chinese medicine (TCM) has been widely used in China. In recent years, a variety of antitumor components extracted from TCM and natural plants have been proven to be effective antitumor agents. Naringin, also called the new hesperidin naringenin glycoside, is a flavanone compound. Our previous studies showed that naringin could significantly inhibit the proliferation of the human ovarian cancer cell line SKOV3 in vitro.5 Naringin also significantly inhibited the proliferation of the cisplatin-resistant human ovarian cancer line SKOV3/CDDP, increasing the sensitivity of SKOV3/CDDP cells to cisplatin treatment. The reversal mechanism might be associated with the NF-κB pathway.6 However, the exact mechanism of naringin in ovarian cancer cells remains largely unknown.
Recent research has indicated that Wnt/β-catenin signaling serves important roles in regulating tumor initiation and progression in all stages of ovarian cancer. In this study, we investigated the effects of naringin on ovarian cancer cells and explored whether the reversal mechanism of cisplatin resistance was related to the Wnt/β-catenin pathway.
Materials and methods
Cells and reagents
The ovarian cancer cell line SKOV3 and the cisplatin-resistant ovarian cancer cell line SKOV3/CDDP were purchased from the Shanghai Academy of Sciences (Shanghai, China). Naringin was provided by the Pharmacology Institute of Nanchang University (Nanchang, China). Cisplatin was purchased from Qilu Pharmaceutical Co., Ltd. (Shandong, China). RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Beijing Soledad Lite-On Technology Co., Ltd. (Beijing, China). Antibodies against β-catenin (cat. no. ab227499), c-Myc (cat. no. ab32072), and cyclin D1 (cat. no. ab134175) were purchased from Abcam (Cambridge, UK). Horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (cat. no. TA130015) was purchased from OriGene Technologies, Inc. (Beijing, China).
SKOV3 and SKOV3/CDDP cells were cultured in RPMI-l640 medium containing 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C under 5% CO2. The medium was replaced every other day, and the cells were passaged every 3 days. Cells in logarithmic phase were used for experimentation.
Naringin preparation
A naringin stock solution (7 mmol/L) was prepared by dissolving 4 mg of naringin in 1 mL of dimethylsulfoxide. Then, working solutions of 80 µmol/L, 40 µmol/L, 20 µmol/L, and 10 µmol/L were prepared by dilution in RPMI-1640.
MTT assay
The cisplatin sensitivity of SKOV3 and SKOV3/CDDP cells was determined by plating cells in logarithmic growth phase in 96-well culture plates at a density of 5 × 103 cells per well. SKOV3-C (cells treated with cisplatin) and SKOV3/CDDP-C (resistant cells treated with cisplatin) indicate that the cells were treated with different concentrations of cisplatin (0, 1, 2, 4, 8, 16, and 32 µg/mL). After 48 hours, the cell proliferation rate and 50% inhibitory concentration (IC50) of SKOV3-C and SKOV3/CDDP-C cells were calculated using the following formula: 1 − experimental group OD value/control group (0 µg/mL cisplatin) OD value × 100%. The cisplatin resistance rate in SKOV3/CDDP-C cells was calculated by the formula: IC50 of cisplatin − the sensitivity of SKOV3/CDDP-C cells ÷ the IC50 SKOV3/CDDP-C cells (cisplatin-resistant). Each test was repeated three times independently.
The inhibitory effects of naringin on SKOV3/CDDP cells were determined by adding 10, 20, 40, and 80 µmol/L naringin to SKOV3/CDDP wells in quintuplicate. SKOV3/CDDP-0 indicates cells that were not treated with cisplatin or naringin. SKOV3/CDDP-N 20 indicates cells that were treated with 20 µmol/L naringin alone. SKOV3/CDDP-C indicated cells treated with different concentrations of cisplatin only. SKOV3/CDDP-CN 20 indicates the SKOV3/CDDP-N 20 cells that were treated with different cisplatin concentrations for 24-, 48-, and 72-hour time points to calculate the inhibitory rate. The naringin concentration that produced a cell growth rate >90% for a given time point was used as the non-cytotoxic dose of naringin (SKOV3/CDDP-N 20 for 48 hours). Each test was repeated three times independently.
We calculated the reversal of cisplatin resistance in SKOV3/CDDP-CN 20 cells after 48 hours of naringin treatment according to the formula: IC50 of SKOV3/CDDP-C cells ÷ IC50 of SKOV/CDDP-CN 20 cells.
Western blotting
Briefly, 8-µg protein samples that were prepared in ice-cold RIPA buffer were separated on 10% SDS gels, and then transferred onto PVDF membranes. The membranes were then blocked in 5% skimmed milk for 2 hours at room temperature, and then incubated overnight with primary antibody solutions for β-catenin, cyclin D1, c-Myc, and tubulin at 4°C. The following day, the membranes were incubated with HRP-conjugated secondary antibodies (1:5000) at room temperature for 2 hours. Immunoreactive bands were developed using the Pierce™ ECL Western Blotting Substrate; anti-tubulin was used as the internal control. Optical density values were analyzed using Image Pro Plus software (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA) to calculate the relative levels of target proteins.
Statistical analysis
Statistical analyses were performed using SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA). The Student's t-test was used for comparisons of the mean values between two groups. Intergroup comparisons were made using one-way ANOVA followed by Fisher's least significant difference test. P < 0.05 indicated a statistically significant difference.
Results
The cisplatin sensitivity of SKOV3 and SKOV3/CDDP cells
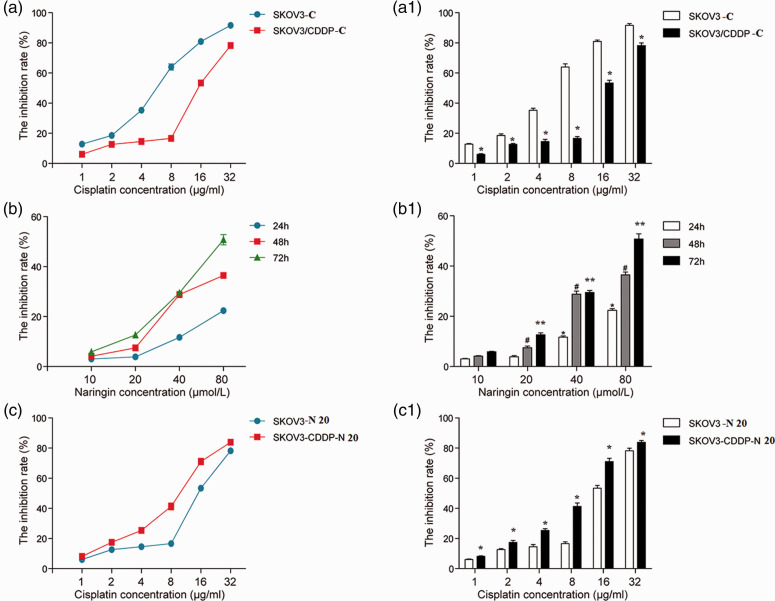
MTT results showed that after incubation with different concentrations of cisplatin for 48 hours, the IC50 was 8.618 µg/mL for SKOV3-C cells and 18.876 µg/mL for SKOV3/CDDP-C cells. The inhibitory rate of SKOV3-C cells was greater than SKOV3/CDDP-C cells under the same incubation time and cisplatin concentration. The inhibitory rate for SKOV3-C vs SKOV3/CDDP-C cells were 16% vs 8%, 19% vs 12%, 36% vs 14%, 62% vs 16%, 81% vs 55%, and 95% vs 77% at cisplatin concentrations of 1, 2, 4, 8, 16, and 32 µg/mL, respectively. These data indicated that SKOV3/CDDP cells were significantly more resistant to cisplatin (*P < 0.05, Figure 1a and a1).
Figure 1.
a and a1: The sensitivity of SKOV3 and SKOV3/CDDP cells to cisplatin.
The inhibitory rate of cisplatin on SKOV3-C cells was significantly greater than SKOV3/CDDP-C cells under the same time and concentration (*P < 0.05).
b and b1: Effects of naringin on the proliferation of SKOV3/CDDP cells.
Different concentrations of naringin had different inhibitory effects on SKOV3/CDDP cells with time and dose dependencies. For SKOV3/CDDP cells at 24 hours, there was no significant difference between 10 and 20 μmol/L naringin (P > 0.05); however, there were significant differences between 20 and 40 μmol/L, 20 and 80 μmol/L, and 40 and 80 μmol/L naringin (*P < 0.05). At 48 and 72 hours, there were significant differences between naringin concentrations above 20 μmol/L (** and #P < 0.05). Compared with the 24-hour time point, there were significant differences at 48 and 72 hours when the naringin concentration was increased to 40 and 80 μmol/L (** and # compared with *, P < 0.05).
c and c1: For SKOV3/CDDP-N 20 cells, the differences were statistically significant (*P < 0.05) compared with SKOV3-N 20 cells (*P < 0.05). The difference of IC50 values between SKOV3/CDDP-CN 20 cells and the SKOV3-N 20 cells was statistically significant (P < 0.05).
SKOV3/CDDP-C: SKOV3/CDDP cells were treated with different concentrations of cisplatin alone. SKOV3/CDDP-CN 20: SKOV3/CDDP-C cells were treated with 20 μmol/L naringin.
Effects of naringin on the proliferation of SKOV3/CDDP cells
The inhibitory effect of naringin on SKOV3/CDDP cells depended on the incubation time and dose (Figure 1b and b1). At the 24-hour time point, there was no significant difference between 10 and 20 µmol/L naringin, but significant differences were observed between 20 and 40 µmol/L, 20 and 80 µmol/L, and 40 and 80 µmol/L naringin (*P < 0.05). At 48 and 72 hours, there were significant differences between all naringin concentrations above 20 µmol/L (** and #P < 0.05). The non-cytotoxic dose of naringin (20 µmol/L for 48 hours; cell growth rate >90%) was used as the optimal concentration and time for subsequent experiments. Compared with the 24-hour time point, there were significant differences at the time points of 48 and 72 hours for naringin concentrations of 40 and 80 µmol/L (** and # compared with *, P < 0.05).
Naringin reversed cisplatin resistance in SKOV3/CDDP cells
With increasing cisplatin concentrations, the inhibitory rate on SKOV3/CDDP-N 20 cells was significantly increased (*P < 0.05) compared with SKOV3-N 20 cells (SKOV3 cells treated with 20 µmol/L naringin) (Figure 1c and c1). The IC50 of SKOV3/CDDP-CN 20 cells was 9.112 µg/mL, while the IC50 of SKOV3-N 20 cells was 18.876 µg/mL; thus, the cisplatin resistant multiple was 2.190, which was statistically significant (P < 0.05), and the naringin reversal multiple (RM) was 2.072.
Effect of naringin on β-catenin, c-Myc, and cyclin D1 protein expression in SKOV3/CDDP cells
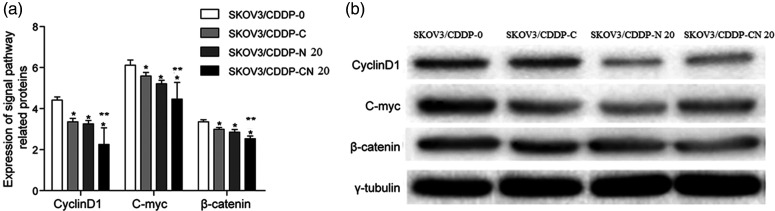
Western blotting results showed that the expression of cyclin D1, c-Myc, and β-catenin proteins decreased in the SKOV3/CDDP-N 20, SKOV3/CDDP-C, and the SKOV3/CDDP-CN 20 groups. These differences were statistically significant compared with the SKOV3/CDDP-0 group (*P < 0.05). The expression of these proteins in the SKOV3/CDDP-CN 20 group was lower than in the SKOV3/CDDP-C and SKOV3/CDDP-N 20 groups (**P < 0.05, Figure 2).
Figure 2.
a: Effects of naringin on β-catenin, c-Myc, and cyclin D1 protein expression in SKOV3/CDDP cells. β-catenin, c-Myc, and cyclin D1 levels in SKOV3/CDDP-C, SKOV3/CDDP-N 20, and SKOV3/CDDP-CN 20 cells were decreased significantly compared with SKOV3/CDDP-0 cells (*P < 0.05). β-catenin, c-Myc, and cyclin D1 expression in SKOV3/CDDP-CN 20 cells was decreased compared with SKOV3/CDDP-C and SKOV3/CDDP-N 20 cells (**P < 0.05).
b: Representative western blots.
SKOV3/CDDP-0: SKOV3/CDDP cells were tested without cisplatin or naringin. SKOV3/CDDP-N 20: SKOV3/CDDP cells were treated with 20 μmol/L naringin alone. SKOV3/CDDP-C: SKOV3/CDDP cells were treated with different concentrations of cisplatin only. SKOV3/CDDP-CN 20: SKOV3/CDDP-C cells were treated with 20 μmol/L naringin.
*P < 0.05 compared with SKOV3/CDDP-0; **P < 0.05 compared with SKOV3/CDDP-C and SKOV3/CDDP-N 20.
Discussion
Chemotherapy resistance of ovarian cancer is the main cause of treatment failure.7 As drug-resistant ovarian cancer has attracted more attention, various Chinese medicines have been suggested as potential anticancer drugs because they have the advantages of reduced toxicity and the ability to reverse resistance.8,9 Naringin is a natural flavonoid compound,10 which primarily exists in the peels of pomelo, grapefruit, orange, and its varieties. A previous study reported that naringin exhibited anti-inflammatory, anti-oxidative stress, hypoglycemic, myocardial protective, and antitumor effects.11–15 However, few studies have examined the effects of naringin on ovarian cancer cells. In our previous study, we found that naringin inhibited the proliferation of the human ovarian cancer cell lines SKOV3 and SKOV3/CDDP in vitro in a time- and dose-dependent manner and that its mechanism may be related to downregulating COX-2 protein expression.5 Naringin also significantly inhibited expression P-gp protein in SKOV3/CDDP cells and reversed their resistance to platinum-based agents. The mechanism of this activity may be related to regulation of the NF-κB signaling pathway.6 In this study, we found that different concentrations of naringin inhibited the proliferation of SKOV3/CDDP cells in a time- and dose-dependent manner. After 20 µmol/L naringin treatment of SKOV3/CDDP-CN for 48 hours, the inhibition rate increased with increasing cisplatin concentrations compared with the 0-µmol/L naringin group (P < 0.05). The IC50 and RM of the combination group were statistically different from the control group. These data indicated that naringin increased the sensitivity of SKOV3/CDDP cells to cisplatin, enhance its cytotoxic effects on ovarian cancer cells, and reversed the cisplatin resistance of SKOV3/CDDP.
The Wnt gene was first isolated in murine breast cancer in 1982, and this signaling pathway regulates many biological processes. Abnormal activation of the Wnt gene is closely related to cardiovascular disease, liver fibrosis, and cancer. The Wnt signaling pathway includes three branches: 1) the canonical Wnt signaling pathway, which is called the Wnt/β-catenin signaling pathway; 2) the Wnt/planner cell polarity pathway; and 3) the Wnt/Ca2+ pathway.16 β-catenin is the key mediator of the canonical Wnt signaling pathway. Abnormal β-catenin expression leads to the inability of cells to degrade β-catenin by phosphorylation and ubiquitination, resulting in its accumulation. Subsequently, β-catenin enters the nucleus, where it activates genes that are crucial for cell division and growth, such as c-Myc and cyclin D1.
A recent study showed that cyclin D1 and c-Myc are important target genes of the Wnt signaling pathway.17 Immunohistochemistry confirmed that abnormal expression of cyclin D1, c-Myc, and β-catenin are correlated with activation of the Wnt signaling pathway.18 When the Wnt signaling pathway is activated, the number of β-catenin molecules entering the nucleus is increased, further activating expression of cyclin D1, c-Myc, and other genes that promote cell proliferation.19 Therefore, these key proteins in the Wnt/β-catenin signaling pathway are often used as drug targets to screen for small molecules that could be used for cancer treatment.20
β-catenin plays an important regulatory role in the development of various human diseases, especially tumors.21 Kildal et al.22 suggested that β-catenin expression is generally higher in ovarian cancer, and that histological type, tumor stage, degree of cancer cell infiltration, and metastasis in ovarian cancer are closely related to abnormal β-catenin expression.
Cyclin D1 is a cell cycle regulator that activates the cyclin E-CDK2 and cyclin A-CDK2 complexes by activating the cyclin dependent kinase 4/6, which promotes cell cycle progression from G1 to S phase.23 Many recent studies have shown that cyclin D1 expression is significantly higher in epithelial ovarian cancer than in benign ovarian and normal ovarian tissues; furthermore, its expression is increased with increased clinical stage and decreased differentiation.24 In advanced ovarian serous cystadenocarcinoma, cyclin D1 overexpression is closely related to poor prognosis and chemotherapeutic resistance.25
c-Myc is a proto-oncogene that is one of the most important members of its gene family. A previous study reported that overexpression of c-Myc is related to the occurrence, invasion, and metastasis of ovarian epithelial cancer.26 In platinum-resistant ovarian cancer, targeted silencing of c-Myc can significantly reduce the growth of ovarian cancer cells and promote apoptosis.27
The mechanism of cisplatin resistance in ovarian cancer cells is complicated. The results obtained in this study are only from experiments performed at the cellular level. In the future, we will conduct animal experiments to further explore the mechanism of cisplatin resistance and its reversal in vivo.
Conclusion
This study investigated the relationship between the naringin-induced reversal of cisplatin resistance in ovarian cancer cells and the expression of Wnt pathway-related proteins. The decreased expression of cyclin D1, c-Myc, and β-catenin in SKOV3/CDDP-CN 20 cells indicated that naringin combined with cisplatin might prevent cell cycle progression from G1 to S phase, enhancing the effect of chemotherapy. Naringin inhibits proliferation by blocking the EMT process, thereby reducing the invasion and metastasis of tumor cells, while also increasing their sensitivity to cisplatin.
Acknowledgements
Not applicable.
Footnotes
Funding: This study was supported by grants from the Traditional Chinese Medicine Research of Jiangxi Provincial Health and Family Planning Commission (No. 2016B079) and the Science and Technology Planning Project of Nanchang City (No. 2017-203-34).
Authors’ contributions
Hong Zhu is the guarantor of the integrity of the entire study and was responsible for study concepts, manuscript preparation, and editing; Xia Zou is responsible for the data and statistical analysis; ShiXin Lin is responsible for the experimental studies and data acquisition; Xin Hu is responsible for the literature research and clinical studies; Jun Gao is responsible for the study design, definition of intellectual content, and manuscript review. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Research involving human participants
This article does not involve patients or animals. This study was approved by the Third Affiliated Hospital Medical Ethics Committee of Nanchang University on June 01, 2017 (2016B079).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Jessmon P, Boulanger T, Zhou W, et al. Epidemiology and treatment patterns of epithelial ovarian cancer. Expert Rev Anticancer Ther 2017; 17: 427–437. [DOI] [PubMed] [Google Scholar]
- 3.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011; 365: 2484–2496. [DOI] [PubMed] [Google Scholar]
- 4.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA 2011; 305: 2295–2303. [DOI] [PubMed] [Google Scholar]
- 5.Song SH, Hu X, Xiong YQ, et al. Effects of Naringin on expression of COX-2 mRNA and protein in human ovarian cancer cell line SKOV3. Chin J Clin Pharmacol Ther 2013; 18: 271–276 (In Chinese). [Google Scholar]
- 6.Zhu H, Gao J, Wang L, et al. In vitro study on reversal of resistance of ovarian cancer cells to cisplatin by Naringin via NF-κB signaling pathway. Exp Ther Med 2018; 15: 2643–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo PE, Fabbro M, Theillet C, et al. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit Rev OncolHematol 2013; 89: 207–216. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Han A, Chen E, et al. The cranberry flavonoids PAC DP-9 and quercetinaglycone induce cytotoxicity and cell cycle arrest and increase cisplatin sensitivity in ovarian cancer cells. Int J Oncol 2015; 46: 1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Wang Y, Lei JC, et al. Sensitisation of ovarian cancer cells to cisplatin by flavonoids from Scutellariabarbata. Nat Prod Res 2014; 28: 683–689. [DOI] [PubMed] [Google Scholar]
- 10.Guihua X, Shuyin L, Jinliang G, et al. Naringin protects ovalbumin-induced airway inflammation in a mouse model of asthma. Inflammation 2016; 39: 891–903. [DOI] [PubMed] [Google Scholar]
- 11.Godwin P, Baird AM, Heavey S, et al. Targeting nuclear factor kappaB to overcome resistance to chemotherapy. Front Oncol 2013; 3: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao AC, Kuo CC, Huang YC, et al. Naringenin inhibits migration of bladder cancer cells through downregulation of AKT and MMP-2. Mol Med Rep 2014; 10: 1531–1536. [DOI] [PubMed] [Google Scholar]
- 13.Yen HR, Liu CJ, Yeh CC. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem Biol Interact 2015; 25: 1–9. [DOI] [PubMed] [Google Scholar]
- 14.Qin L, Jin L, Lu L, et al. Naringenin reduces lung metastasis in a breast cancer resection model. Protein Cell 2011; 2: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Zhu F, Chen H. 6-C-(E-phenylethenyl)-naringenin suppresses colorectal cancer growth by inhibiting cyclooxygenase-1. Cancer Res 2014; 74: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol 2005; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polakis P. Wnt signaling and cancer. Genes Dev 2000; 14: 1837–1851. [PubMed] [Google Scholar]
- 18.Koehler A, Schlupf J, Schneider M, et al. Loss of Xenopus cadherin-11 leads to increased Wnt/beta-catenin signaling and up-regulation of target genes c-myc and cyclin D1 in neural crest. Dev Biol 2013; 383: 132–145. [DOI] [PubMed] [Google Scholar]
- 19.Lim SC, Lee MS. Significance of E-cadherin/β-catenin complex and cyclin D1 in breast cancer. Oncol Rep 2002; 9: 915–928. [PubMed] [Google Scholar]
- 20.Serafino A, Sferrazza G, Colini Baldeschi A, et al. Developing drugs that target the Wnt pathway: recent approaches in cancer and neurodegenerative diseases. Expert Opin Drug Discov 2017; 12: 169–186. [DOI] [PubMed] [Google Scholar]
- 21.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149: 1192–1205. [DOI] [PubMed] [Google Scholar]
- 22.Kildal W, Risberg B, Abeler VM, et al. beta-catenin expression, DNA ploidy and clinicopathological features in ovarian cancer: a study in 253 patients. Eur J Cancer 2005; 41: 1127–1134. [DOI] [PubMed] [Google Scholar]
- 23.Resnitzky D, Gossen M, Bujard H, et al. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 1994; 14: 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue H, Zhao DM, Zheng L. Expression and clinical significance of BORIS, Cyclin D1 protein in epithelial ovarian cancer. Clin J Med Offic 2016; 44: 331–333. [Google Scholar]
- 25.Hashimoto T, Yanaihara N, Okamoto A, et al. Cyclin D1 predicts the prognosis of advanced serous ovarian cancer. Exp Ther Med 2011; 2: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Jiang H, Deng X, et al. Expressions of ZEB2 and C-myc in epithelial ovarian cancer and their clinical significance. Nan Fang Yi Ke Da XueXueBao 2015; 35: 1765–1769. [PubMed] [Google Scholar]
- 27.Reyes-González JM, Armaiz-Peña GN, Mangala LS, et al. Targeting c-MYC in platinum-resistant ovarian cancer. Mol Cancer Ther 2015; 14: 2260–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.