Abstract
Objective:
We aimed to evaluate immune-related adverse events occurring in clinical trials of anti-programmed cell death 1 (PD-1) drugs, compared with control treatments, including chemotherapy, targeted drugs, or placebo. Further we compared the occurrence of immune -related events in patients treated with different anti-PD-1 drugs.
Data Sources:
Randomized controlled trial (RCT) data were sourced from PubMed, Embase, and the Cochrane Central Register of Controlled Trials combined with https://clinicaltrials.gov.
Methods:
Randomized controlled trial of anti-PD-1 drugs compared with control treatments published between January 1, 1970 and March 1,2019, were searched and data on trial patient characteristics, and adverse events extracted, reviewed, and subjected to meta-analysis.
Results:
Eighteen Randomized controlled trials were included in our study. The Randomized controlled trials compared nivolumab (n = 12), pembrolizumab (n = 6), with chemotherapy (n = 13), targeted drugs (n = 2), or placebo (n = 3). Compared with the control group, the risk of any immune-related adverse events in patients treated with anti-PD-1 drugs was increased (RR, 2.65; 95% confidence interval, 1.84–3.83; P < 0.00001). Of the immune-related adverse events, the risk rates of pneumonitis (risk ratio, 2.10; 95% CI, 0.85-5.18), colitis (2.96;1.62-5.38), hypophysitis(4.79;1.54-14.89), hypothyroidism(7.87;5.36-11.57), hyperthyroidism (7.03;4.35-11.34), rash (1.58;0.98-2.54), pruritus (2.28; 1.38-3.76), and hepatitis (9.31;2.18-39.85) were increased by anti-PD-1 drugs. Further, the risk of immune-related adverse events was similar for patients treated with pembrolizumab and nivolumab (P = 0.14).
Conclusions:
In addition to previously reported organ-specific immune-related adverse events, we found that the risk of hyperthyroidism was also increased, in anit-PD-1-treated patients, relative to control treatments. The risk of total immune-related adverse events, was similar for pembrolizumab and nivolumab.
Keywords: anti-PD-1 drugs, immune-related adverse events, systematic review, meta-analysis
Introduction
Immunotherapy, is a type of oncotherapy that boosts physiological defenses against tumors. It functions by impeding or preventing tumor cell growth, enhancing immune system-mediated tumor cell destruction, and preventing cancer from spreading to other parts of the body.
Programmed cell death protein 1(PD-1), an immunoinhibitory receptor of the CD28 family, plays a crucial role in tumor immune escape and is critical for the capacity of the immune system to control cancer growth. There are 2 known ligands for PD-1, programed cell death-ligand 1 and 2 (PD-L1 and PD-L2, also referred to as B7-H1 and B7-DC, respectively). On cells within the tumor microenvironment and in many tumors, PD-L1 is selectively expressed in response inflammatory stimuli. Blocking the interaction between PD-1 and PD-L1 can enhance the immune response in vitro and mediate preclinical antitumor activity.1,2
The side effects of immunotherapy, are collectively referred to as immune-related adverse events (irAE), and are a consequence of aberrant stimulation of the immune system against normal tissues.3 There are 3 types of irAE: organ-specific immune-related adverse events including pneumonitis, hepatitis, and colitis etc; more general immune activation-related adverse events such as fatigue, diarrhea, and rash; and musculoskeletal problems like myalgia, and arthralgia among others.4-7
Based on previous research, to assess the risk of irAE in response to anti-PD-1 drugs, rather than all immune checkpoint inhibitors, compared with control treatments, in randomized controlled trials (RCT), we conducted a systematic review and meta-analysis. Data from both https://ClinicalTrials.gov and published literature were collected. Severe adverse events (grades 3–5) and fatal events were also considered, according to the National Cancer Institute Common Toxicity Criteria.
Material and Methods
This study was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. (a PRISMA checklist is included as Supplementary information S1).
Information Sources and Search Strategy
Before searching, we retrieved all commercial names of anti-PD-1 drugs (up to March 1, 2019) and used Medical Subject Headings to search for all of them to ensure that the search results would not be affected by a lack of terms. We searched PubMed, Embase, and the Cochrane Database for clinical trials published up to March 1, 2019. Two related concepts with the AND operator were used in the search strategy as follow: 1. “nivolumab,” “pembrolizumab,” “Sintilimab,” “Camrelizumab,” “Tislelizumab,” “Cemiplimab,” “PD-1,” or “check inhibitors”; 2. “phase 2 clinical trial,” “phase 3 clinical trial,” “phase 2/3 clinical trial,” “phase 4 clinical trial,” or “RCTs,” to ensure that no eligible studies were overlooked (Box 1). After title/abstract screening by 4 independent investigators (YKW, DJK, CKW and JC) full texts of potentially relevant studies were downloaded and the Methods and Results sections reviewed to determine whether they met the eligibility criteria. When duplicate publications from same study were found, we included only the most recent and complete reports. Then, randomized controlled trial data were sourced from publications on PubMed, Embase, and the Cochrane Central database. To make the collected data more complete, we searched for irAE of anti-PD-1 drugs on https://ClinicalTrials.gov, using the trial numbers in publications. For studies which did not provide complete adverse events information on https://ClinicalTrials.gov, we obtained information from the publication.
Box 1.
PubMed search terms.
Search((((((((((((((((phase 2/3 trial) OR phase 2/3 clinical trial) OR phase II/ III clinical trial) OR phase II/III trial) OR phase 2/3 clinical study) OR phase II/ III clinical study) OR phase 2/3 study) OR phase II/III study) OR phase 2/3 randomized trial) OR phase II/III randomized trial)) OR ((((((Randomized Controlled Trial) OR Clinical Trials, Randomized) OR Trials, Randomized Clinical) OR Controlled Clinical Trials, Randomized) OR randomized controlled trial) OR RCT)) OR ((((((((((((((((Clinical Trials, Phase IV) OR Clinical Trials, Phase 4) OR Drug Evaluation, FDA Phase IV) OR Evaluation Studies, FDA Phase 4) OR Drug Evaluation, FDA Phase 4) OR Evaluation Studies, FDA Phase IV) OR phase 4 clinical trial) OR phase IV clinical trial) OR phase 4 trial) OR phase IV trial) OR phase 4 clinical study) OR phase IV clinical study) OR phase 4 study) OR phase IV study) OR phase 4 randomized trial) OR phase IV randomized trial)) OR (((((((((((((((Clinical Trials, Phase II) OR Evaluation Studies, FDA Phase II) OR Evaluation Studies, FDA Phase 2) OR Drug Evaluation, FDA Phase II) OR Drug Evaluation, FDA Phase 2) OR phase 2 clinical trial) OR phase II clinical trial) OR phase 2 trial) OR phase II trial) OR phase 2 clinical study) OR phase II clinical study) OR phase 2 study) OR phase II study) OR phase 2 randomized trial) OR phase II randomized trial)) OR ((((((((((((((((Clinical Trial, Phase III) OR Clinical Trials, Phase 3) OR Evaluation Studies, FDA Phase III) OR Drug Evaluation, FDA Phase III) OR Drug Evaluation, FDA Phase 3) OR Evaluation Studies, FDA Phase 3) OR phase 3 clinical trial) OR phase III clinical trial) OR phase 3 trial) OR phase III trial) OR phase 3 clinical study) OR phase III clinical study) OR phase 3 study) OR phase III study) OR phase 3 randomized trial) OR phase III randomized trial))) AND (((checkpoint inhibitor) OR PD-1) OR ((((((((((JNJ-63723283) OR PDR001) OR TSR-042) OR BCD-100) OR ((Cemiplimab) OR REGN-2810)) OR ((Tislelizumab) OR BGB-A317)) OR ((Camrelizumab) OR SHR-1210)) OR ((IBI308) OR Sintilimab)) OR (((((pembrolizumab) OR pembrolizumab) OR lambrolizumab) OR keytruda) OR SCH 900475)) OR (((((((((((((nivolumab) OR Nivolumab) OR Opdivo) OR ONO-4538) OR ONO 4538) OR ONO4538) OR MDX-1106) OR MDX 1106) OR MDX1106) OR BMS-936558) OR BMS 936558) OR BMS936558) OR NIVO))
Study Selection and Eligibility Criteria
The aim of our study was to assess the risk of irAE following the use of anti-PD-1 drugs, compared with control groups in patients with cancer, and to compare the occurrence of irAE in patients receiving different kinds of anti-PD-1 drugs. Reviews, editorials, conference, correspondence, phase 1 trials, and nonrandomized studies were excluded. Studies that met the following criteria were included in the analysis: (1) study type was prospective phase 3 RCTs involving patients with cancer;(2)participants were patients diagnosed with cancer, regardless of age, ethnicity, sex and geographical region;(3) interventions were random assignment of participants to anti-PD-1 drugs; (4)control group included patients receiving chemotherapy, targeted drugs, or placebo; (5)outcomes were available data regarding irAEs and the number of irAE.
Data Collection Process
Data were extracted (YKW, DJK and CKW) and verified (YKW and JC) by independent reviewers. For each study, the following information was extracted: year of publication, first author, types of cancer in anti-PD1-treated and control groups, name of anti-PD-1 drugs, number of patients in each group, and number of all adverse events (data are available in Supplementary information S2). According to previous study6 and preliminary analysis of the data collected here(Supplementary information S2), the primary outcomes of the review were organ specific irAE(pneumonitis, hepatitis, hypophysitis, hypothyroidism, hyperthyroidism and colitis)and general irAE(rash, pruritus). The secondary outcome was associated musculoskeletal problems (arthritis and myalgia).
Statistical Analyses
Data were pooled to compare the risks of irAE between patients receiving anti-PD-1 drugs and control groups. Confidence intervals for the risk ratio (RR)8 were calculated using the Woolf method. Two models, meta-analysis with fixed-effects (Mantel–Haenszel method) and random-effects (Der Simonian and Laird method), were considered based on the heterogeneity of the included studies. Before we pooled data, we evaluated the heterogeneity of all studies. Heterogeneity among studies was assessed using Cochran’s Q statistic. Inconsistency was evaluated using the I2 statistic, which measures the total percentage of variation across studies due to heterogeneity rather than chance. An I2 values of 0% indicates no observed heterogeneity, while values between 0% and 100% show increasing heterogeneity. Where I2 values were <50% heterogeneity (P value > 0.1), pooled RR and 95% confidence interval (CI) were estimated using a fixed effects model and a random effects model was used when the assumption of homogeneity was considered invalid (P value < 0.1) and I2 >50%. We added a standard continuity correction of 0.5 to each cell, when zero event studies were included.9 If sufficient studies assessing nivolumab and pembrolizumab were available, we conducted subgroup analyses to assess the occurrence of irAE in patients treated with different anti-PD-1 drugs. We used funnel plots, Begg’s rank test and Egger’s regression test10 to assess publication bias. All statistical analyses were conducted using Review Manager 5.3 (Copenhagen, Denmark), Stata 15 and Microsoft Office 2019.
Results
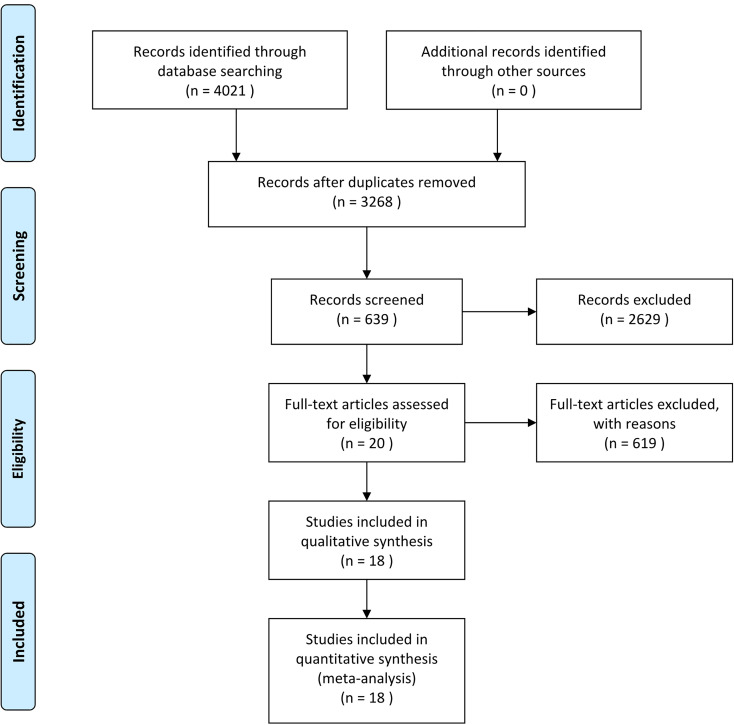
Initially, we identified a total of 4021citations through database searches and other sources. Of these, 18 finally underwent full text review and 19 unique trials were included for quantitative synthesis and meta-analysis. The other studies were excluded for the reasons described the flow diagram presented in Figure 1.
Figure 1.
Flow diagram according to RISMA 2009.
Quality of Included Studies
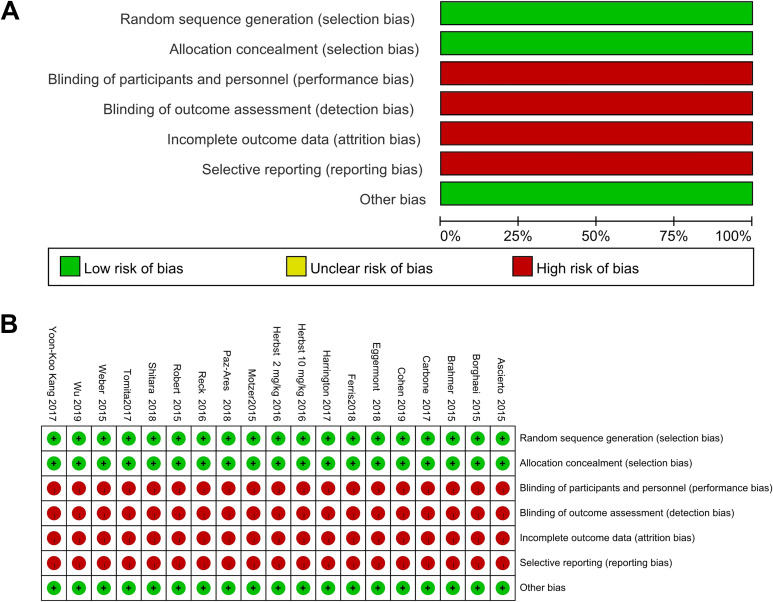
Although all included studies were RCT, the primary endpoint was survival. As adverse events are reported by clinicians who directly care for patients, studies were unmasked. Furthermore, since included studies were not designed mainly to assess adverse events, collection of adverse events information was poorly described; therefore, we considered all studies at high risk of bias with regard to blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective outcome reporting (Figure 2).
Figure 2.
Graph summarizing bias risk and applicability concerns.
Study Characteristics
Of the 18 included RCTs,13 compared anti-PD-1 drugs with chemotherapy, 2 with targeted drugs, and 2 with placebo as single agents. In one trial, anti-PD-1 drug plus chemotherapy was compared with placebo plus chemotherapy. Seven RCTs were conducted in patients with non-small cell lung cancer (NSCLC), 4 in patients with melanoma, 3 in patients with carcinoma of the head and neck, and 2 each in patients with renal cell cancer and gastric or gastro-esophageal junction cancer. (Table 1).
Table 1.
Characteristics of Studies Included in the Meta-Analysis.
| Cancer | Drug | Colitis | Pneumonitis | Hypothyroidism | Hyperthyroidism | Rash | Pruritus | Hepatitis | Hypophysitis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AE | Total | AE | Total | AE | Total | AE | Total | AE | Total | AE | Total | AE | Total | AE | Total | |||
| Robert 2015 | Melanoma | Nivolumab | 0 | 206 | 0 | 206 | 0 | 206 | 0 | 206 | 31 | 206 | 35 | 206 | 0 | 206 | 0 | 206 |
| Chemotherapy | 0 | 205 | 0 | 205 | 0 | 205 | 0 | 205 | 6 | 205 | 11 | 205 | 0 | 205 | 0 | 205 | ||
| Ascierto 2015 | Melanoma | Nivolumab | 0 | 206 | 0 | 206 | 13 | 206 | 0 | 206 | 38 | 206 | 49 | 206 | 0 | 206 | 0 | 206 |
| Chemotherapy | 0 | 205 | 0 | 205 | 2 | 205 | 0 | 205 | 6 | 205 | 11 | 205 | 0 | 205 | 0 | 205 | ||
| Borghaei 2015 | Non-small cell | Nivolumab | 0 | 287 | 0 | 287 | 0 | 287 | 0 | 287 | 0 | 287 | 0 | 287 | 0 | 287 | 0 | 287 |
| lung cancer | Chemotherapy | 0 | 268 | 0 | 268 | 0 | 268 | 0 | 268 | 0 | 268 | 0 | 268 | 0 | 268 | 0 | 268 | |
| Wu 2019 | Non-small cell | Nivolumab | 0 | 337 | 0 | 337 | 0 | 337 | 0 | 337 | 39 | 337 | 0 | 337 | 0 | 337 | 0 | 337 |
| lung cancer | Chemotherapy | 0 | 156 | 0 | 156 | 0 | 156 | 0 | 156 | 4 | 156 | 0 | 156 | 0 | 156 | 0 | 156 | |
| Brahmer 2015 | Non-small cell | Nivolumab | 0 | 131 | 6 | 131 | 0 | 131 | 0 | 131 | 5 | 131 | 0 | 131 | 0 | 131 | 0 | 131 |
| lung cancer | Chemotherapy | 0 | 129 | 0 | 129 | 0 | 129 | 0 | 129 | 8 | 129 | 0 | 129 | 0 | 129 | 0 | 129 | |
| Carbone 2017 | Non-small cell | Nivolumab | 0 | 267 | 0 | 267 | 0 | 267 | 0 | 267 | 26 | 267 | 0 | 267 | 0 | 267 | 0 | 267 |
| lung cancer | Chemotherapy | 0 | 263 | 0 | 263 | 0 | 263 | 0 | 263 | 15 | 263 | 0 | 263 | 0 | 263 | 0 | 263 | |
| Weber 2015 | Melanoma | Nivolumab | 0 | 268 | 0 | 268 | 0 | 268 | 0 | 268 | 0 | 268 | 43 | 268 | 0 | 268 | 0 | 268 |
| Chemotherapy | 0 | 102 | 0 | 102 | 0 | 102 | 0 | 102 | 0 | 102 | 2 | 102 | 0 | 102 | 0 | 102 | ||
| Harrington 2017 | Carcinoma of the | Nivolumab | 0 | 240 | 0 | 240 | 0 | 240 | 0 | 240 | 0 | 240 | 0 | 240 | 0 | 240 | 0 | 240 |
| head and neck | Chemotherapy | 0 | 121 | 0 | 121 | 0 | 121 | 0 | 121 | 0 | 121 | 0 | 121 | 0 | 121 | 0 | 121 | |
| Ferris 2018 | Carcinoma of the | Nivolumab | 0 | 236 | 0 | 236 | 0 | 236 | 0 | 236 | 18 | 236 | 17 | 236 | 17 | 236 | 0 | 236 |
| head and neck | Chemotherapy | 0 | 111 | 0 | 111 | 0 | 111 | 0 | 111 | 5 | 111 | 0 | 111 | 0 | 111 | 0 | 111 | |
| Yoon-Koo Kang 2017 | Gastric or gastro- | Nivolumab | 2 | 330 | 1 | 330 | 1 | 330 | 2 | 330 | 19 | 330 | 30 | 330 | 0 | 330 | 10 | 330 |
| esophageal junction cancer | Placebo | 0 | 161 | 0 | 161 | 0 | 161 | 0 | 161 | 5 | 161 | 9 | 161 | 0 | 161 | 1 | 161 | |
| Motzer 2015 | Renal cell cancer | Nivolumab | 0 | 406 | 16 | 406 | 0 | 406 | 0 | 406 | 0 | 406 | 57 | 406 | 0 | 406 | 0 | 406 |
| Target drugs | 0 | 397 | 58 | 397 | 0 | 397 | 0 | 397 | 0 | 397 | 39 | 397 | 0 | 397 | 0 | 397 | ||
| Tomita 2017 | Renal cell cancer | Nivolumab | 0 | 37 | 0 | 37 | 0 | 37 | 0 | 37 | 0 | 37 | 3 | 37 | 2 | 37 | 0 | 37 |
| Target drugs | 0 | 26 | 5 | 26 | 0 | 26 | 0 | 26 | 0 | 26 | 6 | 26 | 7 | 26 | 0 | 26 | ||
| Herbst 2 mg/kg 2016 | Non-small cell | Pembrolizumab (2 mg/kg) | 4 | 339 | 16 | 339 | 28 | 339 | 12 | 339 | 29 | 339 | 0 | 339 | 0 | 339 | 1 | 339 |
| lung cancer | Chemotherapy | 0 | 309 | 6 | 309 | 1 | 309 | 3 | 309 | 14 | 309 | 0 | 309 | 0 | 309 | 0 | 309 | |
| Herbst 10 mg/kg 2016 | Non-small cell | Pembrolizumab(10mg/kg) | 2 | 343 | 15 | 343 | 28 | 343 | 20 | 343 | 44 | 343 | 0 | 343 | 0 | 343 | 1 | 343 |
| lung cancer | Chemotherapy | 0 | 309 | 6 | 309 | 1 | 309 | 3 | 309 | 14 | 309 | 0 | 309 | 0 | 309 | 0 | 309 | |
| Cohen 2019 | Carcinoma of the | Pembrolizumab | 2 | 246 | 10 | 246 | 33 | 246 | 5 | 246 | 19 | 246 | 0 | 246 | 0 | 246 | 0 | 246 |
| head and neck | Chemotherapy | 1 | 234 | 3 | 234 | 2 | 234 | 1 | 234 | 34 | 234 | 0 | 234 | 0 | 234 | 0 | 234 | |
| Reck 2016 | Non-small cell | Pembrolizumab | 3 | 154 | 9 | 154 | 12 | 154 | 14 | 154 | 0 | 154 | 0 | 154 | 0 | 154 | 1 | 154 |
| lung cancer | Chemotherapy | 0 | 150 | 1 | 150 | 2 | 150 | 2 | 150 | 0 | 150 | 0 | 150 | 0 | 150 | 0 | 150 | |
| Shitara 2018 | Gastric or gastro- | Pembrolizumab | 3 | 294 | 8 | 294 | 23 | 294 | 12 | 294 | 0 | 294 | 0 | 294 | 4 | 294 | 4 | 294 |
| esophageal junction cancer | Chemotherapy | 4 | 276 | 0 | 276 | 1 | 276 | 1 | 276 | 0 | 276 | 0 | 276 | 0 | 276 | 0 | 276 | |
| Eggermont 2018 | Melanoma | Pembrolizumab | 19 | 509 | 17 | 509 | 73 | 509 | 52 | 509 | 82 | 509 | 90 | 509 | 9 | 509 | 0 | 509 |
| Placebo | 3 | 502 | 6 | 502 | 14 | 502 | 6 | 502 | 54 | 502 | 51 | 502 | 1 | 502 | 0 | 502 | ||
| Paz Ares 2018 | Non-small cell | Pembrolizumab | 7 | 278 | 18 | 278 | 22 | 278 | 20 | 278 | 0 | 278 | 0 | 278 | 5 | 278 | 3 | 278 |
| lung cancer | Placebo | 4 | 280 | 6 | 280 | 5 | 280 | 2 | 280 | 0 | 280 | 0 | 280 | 0 | 280 | 0 | 280 | |
Patients
A total of 9318 patients were randomized in the 18 phase 3 RCTs included in this meta-analysis. Of these, in trials of nivolumab,2951 patients were assigned to nivolumab, 1560 to chemotherapy, 161 to placebo, and 423 to targeted drugs (everolimus). Further, in trials of pembrolizumab, 2163 patients were assigned to pembrolizumab, 1278 to chemotherapy, 502 to placebo, 280 to placebo plus chemotherapy, and no patients to targeted drugs (Table 1). The performance status of all patients in these studies was between 0 and 2. The safety population, which included all patients who were exposed to at least 1 dose of the treatment, consisted of 9318 patients (Anti-PD-1 drugs,5114; control,4204) with NSCLC (4000), gastric or gastro-esophageal junction cancer (1061), carcinoma of the head and neck (1188), renal cell cancer (866), and melanoma (2293) (Table 1).
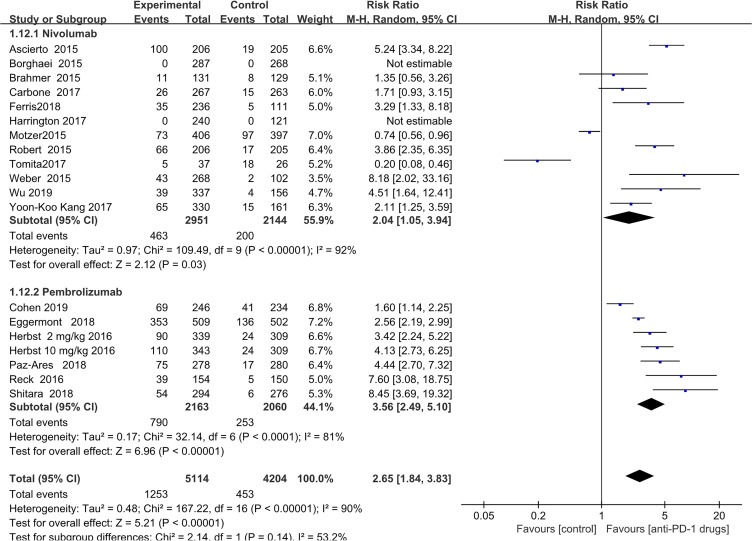
The following organ specific irAE were recorded pneumonitis (n = 116), colitis(n = 42), hypophysitis(n = 21), hypothyroidism (n = 233), hyperthyroidism (n = 138) and hepatitis patients(n = 18). General immune activation-related adverse events were recorded rash (n = 394), pruritus (n = 324). (Table 1). Compared with the control group, the risk of (any irAE other than musculoskeletal problems) in patients treated with anti-PD-1 drugs was increased (RR, 2.65;95%CI 1.84-3.83; P < 0.00001); Further, the risk of irAE was similar for patients treated with pembrolizumab and nivolumab (P = 0.14) (Figure 3).
Figure 3.
Forest plot of total immune-related adverse events.
Immune-Related Adverse Events
Organ-specific immune-related adverse events.
Pneumonitis
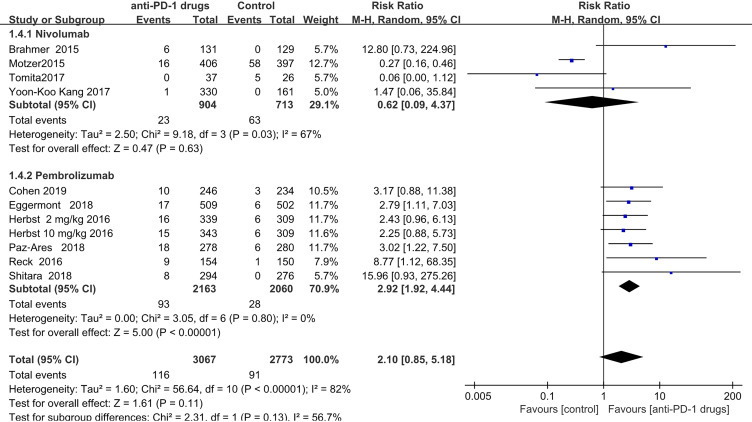
Pneumonitis was observed in both the anti-PD-1 (n = 116 patients) and control (n = 91patients) groups. The RR values obtained for the studies ranged from 0.06 (Tomita 2017) to 15.96 (Shitara 2018). The overall pooled RR, obtained by meta-analysis using a random-effects model was 2.10(95% CI,0.85–5.18; P = 0.11), indicating no significant increased risk. As the observed heterogeneity was mainly attributable to the studies of nivolumab, we separately used a fixed-effects model to analyze the occurrence of pneumonitis in patients treated with pembrolizumab. Patients treated with pembrolizumab had a significantly increased risk of pneumonitis (pooled RR = 3.12; 95% CI,2.06–4.73; P < 0.00001) (Supplementary information S3, Figure 1); however, there was no significant risk associated with nivolumab treatment (pooled RR = 0.62; 95% CI 0.09–4.37; P = 0.63) (Figure 4).
Figure 4.
Forest plot of pneumonitis.
Colitis
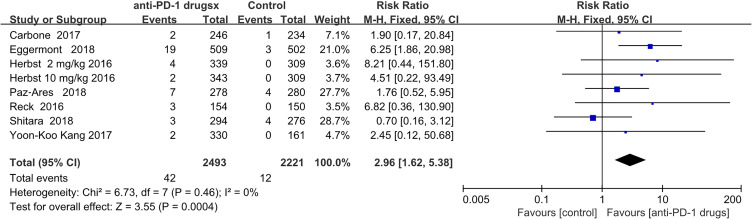
Colitis was diagnosed in 42 and 12 patients in the anti-PD-1 and control groups, respectively. The pooled RR was 2.96 (95% CI 1.62–5.38; P = 0.0004), indicating a significantly increased risk. Related to anti-PD1 treatment, since there was only 1 study of nivolumab where colitis was recorded, we did not conduct a subgroup analysis (Figure 5).
Figure 5.
Forest plot of colitis.
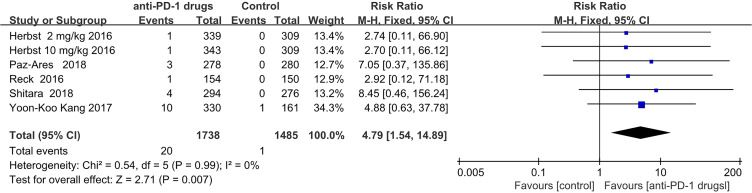
Hypophysitis
Hypophysitis events occurred almost exclusively in the anti-PD-1 group (20 of 21 total events); with one occurrence in a control group. There was significantly increased risk for patients who received anti-PD-1therapy (RR = 4.79; 95% CI,1.54–14.89; P = 0.007). As there was only 1 study of nivolumab that recorded hypophysitis, we did not perform a subgroup analysis. (Figure 6).
Figure 6.
Forest plot of hypophysitis.
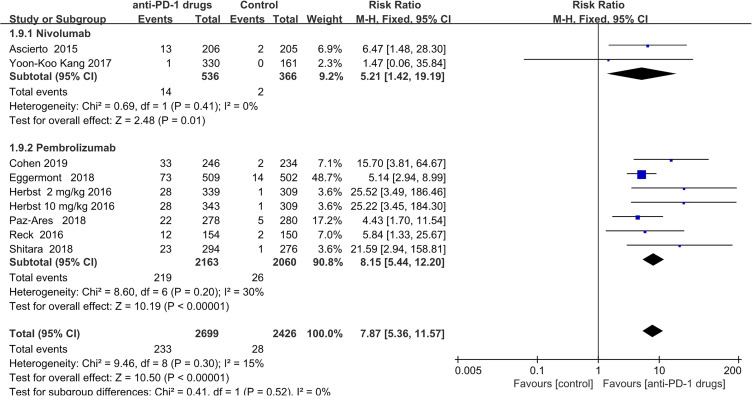
Hypothyroidism
Hypothyroidism was reported in patients treated with both anti-PD-1 (n = 233) and in controls (n = 28). Patients who received anti-PD-1 therapy had a significantly increased risk of hypothyroidism (pooled RR = 7.87; 95% CI, 5.36–11.57; P < 0.00001). Subgroup analysis showed that 14 patients developed hypothyroidism in the nivolumab treated group, and 2 in the control group (pooled RR = 5.21;95% CI,1.42–19.19; P = 0.01), while219 patients developed hypothyroidism in the pembrolizumab treated group versus 26 in the control group (pooled RR = 8.15;95% CI 5.44–12.20; P < 0.0001) (Figure 7).
Figure 7.
Forest plot of hypothyroidism.
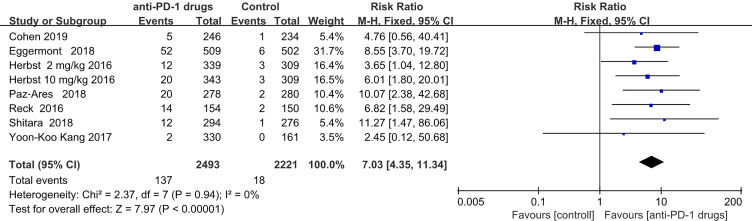
Hyperthyroidism
Hyperthyroidism was diagnosed in 137 anti-PD-1 treated and 18 control group patients. Hence, patients who received anti-PD-1 therapy were at significant risk of hyperthyroidism (RR = 7.03; 95% CI 4.35–11.34; P < 0.00001).As only 1 study of nivolumab recorded hyperthyroidism, we did not perform subgroup analysis .No association of hyperthyroidism with anti-PD-1 treatment has previously been reported (Figure 8).
Figure 8.
Forest plot of hyperthyroidism.
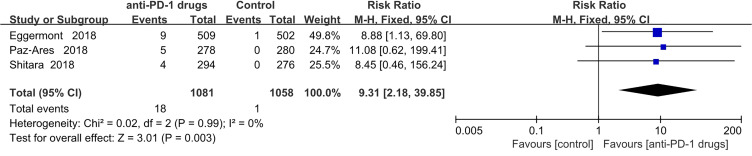
Hepatitis
Hepatitis was observed 18 patients treated with anti-PD-1 and 1 control group patients. The pooled RR was 9.31 (95% CI 2.18–39.85; P = 0.003). All patients with recorded hepatitis were in the pembrolizumab group and none in the nivolumab group. (Figure 9).
Figure 9.
Forest plot of hepatitis.
General immune activation-related adverse events.
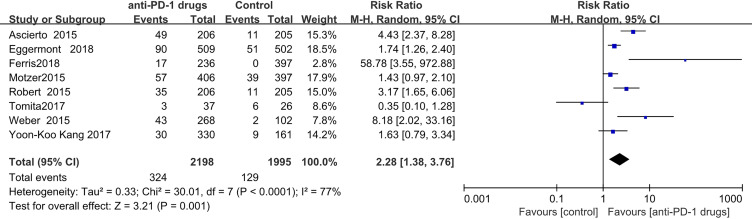
Pruritus
Pruritis was recorded in324 patients receiving anti-PD-1 treatment and 128 administered control treatments. The estimated RR obtained by meta-analysis using a random-effects model, was 2.28 (95% CI,1.38-3.76 P < 0.0001). Since there was only 1 study of pembrolizumab that recorded pruritus, we did not conduct a subgroup analysis. (Figure 10)
Figure 10.
Forest plot of pruritus.
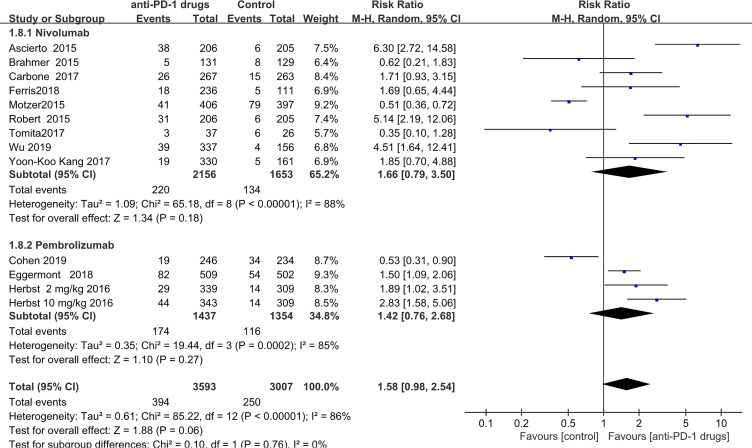
Rash
Rash was observed in 394 patients receiving anti-PD-1 treatment and 250 control group patients. The pooled RR, according to random-effects model meta-analysis was 1.58 (pooled 95% CI,0.98–2.54; P = 0.06). In subgroup analysis, 220 patients were diagnosed with rash in the nivolumab treated group and 134 in the control groups (RR = 1.66; 95% CI 0.79–3.50; P = 0.18), indicating no significant increase in the risk of rash events in the nivolumab-treated subgroup. Further, no statistically significant risk was detected for patients who received pembrolizumab (pooled RR = 1.42; 95% CI 0.76–2.68; P = 0.27) .These results are not consistent with previously published data6(Figure 11).
Figure 11.
Forest plot of rash.
Musculoskeletal problems
There were 133 and 144 patients with recorded musculoskeletal problems. The pooled RR, calculated using random-effects model meta-analysis, was 0.89 (95% CI,0.37–2.21; P = 0.78), hence, there was no significant risk of musculoskeletal problems (Supplementary information S3, Figure 2).11-30
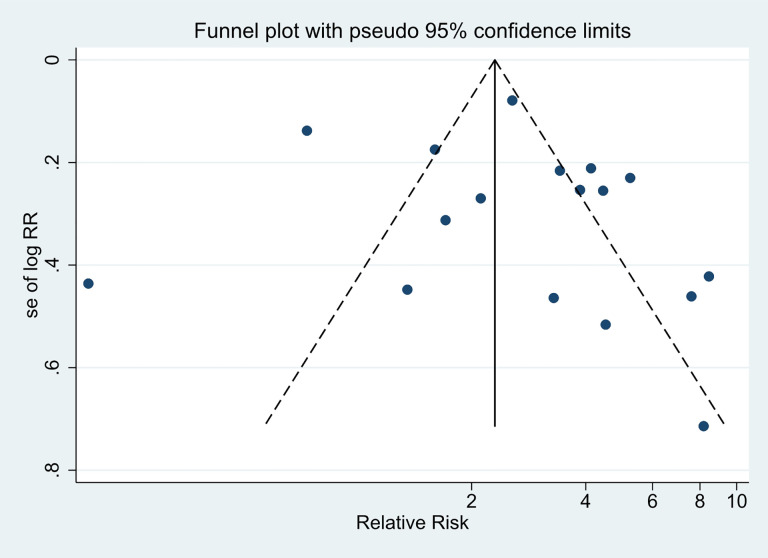
Publication Bias
The distribution of the irAE on both sides of the funnel plot is symmetrical. Further Begg’s (P = 0.967) and Egger’s (P = 0.493;95%CI,-2.077279-4.12382) suggested that there was no publication bias. (Figure 12 funnel plot generated using Stata 15).
Figure 12.
Funnel plot to assess potential publication bias.
Discussion
In this study, we completed a systematic comparison of immune-related adverse events between patients receiving anti-PD-1 drugs and other treatments, using data from 18 RCTs, including 9318 treated patients. We found the that patients treated with anti-PD-1 drugs had significantly higher risks for organ-specific irAE(hepatitis, hypophysitis, hypothyroidism, hyperthyroidism and colitis) than those in control groups; however, the data included in the statistical analysis were not serious adverse events.
Mechanisms of the action of anti-PD-1 drugs involve enhancement of patient immune function, through active or passive methods.31 We conducted a subgroup analysis of total irAE between pembrolizumab and nivolumab, and found that the risk was similar between the 2 drugs. It can help clinicians who choose the 2 types of anti-PD-1drugs based on their therapeutic effects, rather than with the aim of reducing irAE in the future.
We found that risk of hyperthyroidism, was a significantly and markedly increased in patients receiving anti-PD-1 drugs. Based on the findings of a previous study32 combined with the results of this review, we consider anti-PD-1drug have bidirectional effects on thyroid function, as they can cause hypothyroidism or hyperthyroidism. This finding could help clinicians to identify and correctly address thyroid function related irAE when treating patients with anti-PD-1 drugs.
Our results regarding the risk of rash were inconsistent with those of a previous study.6 The risk of rash was not increased. Since we included more anti-PD-1 drug studies, any differences in the results presented here with those of previous reviews could be attributed to that factor. Furthermore, we found that the risk of musculoskeletal problems was similar between anti-PD-1drugs and other therapies. We speculate that the researchers who conducted the RCT may not have been fully informed about the risk of musculoskeletal problems as irAE, leading to inaccurate diagnosis and recording of these events.
Study Strengths and Limitations
The strengths of this study are that all included studies were randomized controlled trial and that it was focused on anti-PD-1 drugs, rather than all immune checkpoint inhibitors, making the studies included in this review less heterogenous than those in previously published analyses, and our results more reliable. In addition, we performed subgroup analyses of patients treated with pembrolizumab and nivolumab, to increase the specificity of our irAE data. Moreover, compared with a previous review,6 this review updated data from previous RCT and data from additional RCT conducted over the last 2 years.
Conclusion
Consistent with previous reports, the risk of organ-specific irAE in patients treated with anti-PD-1 drugs was higher than that for those administered control treatments. The results of this review, demonstrated that, compared with control groups, the risk of hyperthyroidism is also increased in patients receiving anti-PD-1 treatment; however, the risk of rash as a general immune activation-related adverse event was not increased. The overall risk of irAE was similar for patients treated with pembrolizumab and nivolumab.
Supplemental Material
Supplemental Material, S2.Data_of_all_adverse_events for A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials by Yukun Wang, Dejiu Kong, Chaokun Wang, Jing Chen, Jing Li, Zhiwei Liu, Xinyang Li, Ziming Wang, Ge Yao and Xinshuai Wang in Technology in Cancer Research & Treatment
Supplemental Material, S3.Fig.1_Forest_ofpneumonitis_in_patients_treated_with_pembrolizumab for A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials by Yukun Wang, Dejiu Kong, Chaokun Wang, Jing Chen, Jing Li, Zhiwei Liu, Xinyang Li, Ziming Wang, Ge Yao and Xinshuai Wang in Technology in Cancer Research & Treatment
Supplemental Material, S3.Fig.2_Forest_plot_of_musculoskeletal_problems for A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials by Yukun Wang, Dejiu Kong, Chaokun Wang, Jing Chen, Jing Li, Zhiwei Liu, Xinyang Li, Ziming Wang, Ge Yao and Xinshuai Wang in Technology in Cancer Research & Treatment
Supplemental Material, S4.Editorial_Certificate for A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials by Yukun Wang, Dejiu Kong, Chaokun Wang, Jing Chen, Jing Li, Zhiwei Liu, Xinyang Li, Ziming Wang, Ge Yao and Xinshuai Wang in Technology in Cancer Research & Treatment
Abbreviations
- Aes
adverse events
- Akt
protein kinase B (PKB), also known as Akt
- irAE
immune-related adverse events
- PD 1
programed cell death protein 1
- PD-L1
programed death-ligand 1
- PD-L2
programed death-ligand 2
- CI
confidence interval
- NSCLC
non-small cell lung cancer
- PRISMA
preferred reporting items for systematic reviews and meta-analyses
- RCT
randomized controlled trial
- RR
relative risk.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Our study did not require an ethical board approval because it is a systematic review and meta-analysis and it did not contain human or animal trials.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Xinshuai Wang  https://orcid.org/0000-0002-9566-4891
https://orcid.org/0000-0002-9566-4891
References
- 1. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zuazo M, Arasanz H, Fernández-Hinojal G, et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol Med. 2019;11(7):e10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gianchecchi E, Fierabracci A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in treg development and their involvement in autoimmunity onset and cancer progression. Front Immunol. 2018;9:2374 doi:10.3389/fimmu.2018.02374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdel-Rahman O, ElHalawani H, Fouad M. Risk of gastrointestinal complications in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2015;7(11):1213–1227. doi:10.2217/imt.15.87 [DOI] [PubMed] [Google Scholar]
- 5. Abdel-Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther Adv Respir Dis. 2016;10(3):183–193. doi:10.1177/1753465816636557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360(undefined):k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdel-Rahman O, ElHalawani H, Fouad M. Risk of elevated transaminases in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Expert Opin Drug Saf. 2015;14(10):1507–1518. doi:10.1517/14740338.2015.1085969 [DOI] [PubMed] [Google Scholar]
- 8. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16 doi:10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedrich JO, Adhikari NKJ, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7(1):5 doi:10.1186/1471-2288-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–794. doi:10.1111/biom.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eggermont AMM, Blank CU, Mandala M, Long GV. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi:10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 12. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi:10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). 2017;390(10111):2461–2471. doi:10.1016/s0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 14. Shukla S, Nanavaty RR, Gupta S, et al. Nivolumab in previously untreated melanoma without BRAF mutation. Cell Death Dis. 2015;372(4):320–330. doi:10.1038/cddis.2014.500. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 15. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Clinical Trial, Phase III; Journal Article; Multicenter Study; Randomized Controlled Trial; Research Support, Non-U.S. Gov’t. Lancet Oncol. 2015;16(4):375–384. doi:10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 16. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867–875. doi:10.1016/j.jtho.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 17. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. Clinical Trial, Phase III; Comparative Study; Journal Article; Multicenter Study; Randomized Controlled Trial; Research Support, Non-U.S. Gov’t. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomita Y, Fukasawa S, Shinohara N, et al. Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup analysis from the CheckMate 025 study. Clinical Trial, Phase III; Journal Article; Randomized Controlled Trial. Jpn J Clin Oncol. 2017;47(7):639-646. doi:10.1093/jjco/hyx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi:10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrington KJ, Ferris RL, Blumenschein G, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18(8):1104-1115. doi:10.1016/s1470-2045(17)30421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45-51. doi:10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. Clinical Trial, Phase III; Journal Article; Multicenter Study; Randomized Controlled Trial; Research Support, Non-U.S. Gov’t. N Engl J Med. 2018;379(21):2040-2051. doi:10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 24. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi:10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 25. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Journal Article; Multicenter Study; Randomized Controlled Trial; Research Support, Non-U.S. Gov’t. Lancet (London, England). 2016;387(10027):1540-1550. doi:10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 26. Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;393(10167):156–167. doi:10.1016/s0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 27. Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England). 2018;392(10142):123–133. doi:10.1016/s0140-6736(18)31257 -1 [DOI] [PubMed] [Google Scholar]
- 28. Kiyota N, Hasegawa Y, Takahashi S, et al. A randomized, open-label, Phase III clinical trial of nivolumab vs. therapy of investigator’s choice in recurrent squamous cell carcinoma of the head and neck: a subanalysis of Asian patients versus the global population in checkmate 141. Oral Oncol. 2017;73:138–146. doi:10.1016/j.oraloncology.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 29. Kato K, Satoh T, Muro K, et al. A subanalysis of Japanese patients in a randomized, double-blind, placebo-controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2). Gastric Cancer. 2019;22(2):344–354. doi:10.1007/s10120-018-0899-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ascierto PA, Long GV, Robert C, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019;5(2):187–194. doi:10.1001/jamaoncol.2018.4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Escors D, Gato-Cañas M, Zuazo M, et al. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct Target Ther. 2018;3(undefined):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Velasco G, Je Y, Bossé D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312–318. doi:10.1158/2326-6066.cir-16-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, S2.Data_of_all_adverse_events for A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials by Yukun Wang, Dejiu Kong, Chaokun Wang, Jing Chen, Jing Li, Zhiwei Liu, Xinyang Li, Ziming Wang, Ge Yao and Xinshuai Wang in Technology in Cancer Research & Treatment
Supplemental Material, S3.Fig.1_Forest_ofpneumonitis_in_patients_treated_with_pembrolizumab for A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials by Yukun Wang, Dejiu Kong, Chaokun Wang, Jing Chen, Jing Li, Zhiwei Liu, Xinyang Li, Ziming Wang, Ge Yao and Xinshuai Wang in Technology in Cancer Research & Treatment
Supplemental Material, S3.Fig.2_Forest_plot_of_musculoskeletal_problems for A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials by Yukun Wang, Dejiu Kong, Chaokun Wang, Jing Chen, Jing Li, Zhiwei Liu, Xinyang Li, Ziming Wang, Ge Yao and Xinshuai Wang in Technology in Cancer Research & Treatment
Supplemental Material, S4.Editorial_Certificate for A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-PD-1 Drugs in Randomized Controlled Trials by Yukun Wang, Dejiu Kong, Chaokun Wang, Jing Chen, Jing Li, Zhiwei Liu, Xinyang Li, Ziming Wang, Ge Yao and Xinshuai Wang in Technology in Cancer Research & Treatment