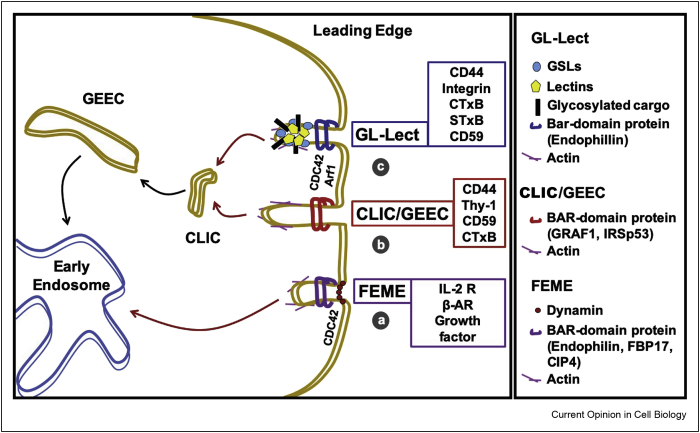
Figure 1.
Clathrin-independent endocytic processes. Schematic representation of the leading edge of a migratory cell where processes of clathrin-independent endocytosis mainly operate, while the clathrin pathway remains unpolarized. (a) FEME, fast endophilin-mediated endocytosis. FEME relies on the BAR-domain protein family member endophilin and other BAR domain proteins. This endocytic process is used by cargo proteins such as IL-2R, β-adrenergic receptor (β-AR), and growth factors. (b) CLIC/GEEC endocytosis. Clathrin-independent carriers are short tubular often crescent-shaped endocytic carriers that mature into glycophosphatidylinositol-anchored protein-enriched early endocytic compartments. CLIC/GEEC endocytosis is regulated by the GTPase activating factor GRAF1 and the small GTPase CDC42, among others, for the cellular uptake of cargoes like the hyaluronic acid receptor CD44, the GPI-anchored proteins CD59 and Thy-1, and the bacterial cholera toxin. (c) GL-Lect hypothesis. Molecular hypothesis according to which sugar-binding proteins of pathogenic (e.g. the GSL-binding B-subunits of Shiga and cholera toxin, STxB and CTxB, respectively, or the GSL-binding VP1 capsid protein of SV40) or cellular origin (e.g. galectins) reorganize glycolipids to which they bind in a way such as to drive the biogenesis of tubular endocytic pits from which CLICs are generated for the cellular uptake of pathogens (e.g. SV40 virus), pathogenic products (e.g. Shiga and cholera toxins), or cellular proteins (e.g. CD44, integrins, CD59) that are recruited by the galectins.