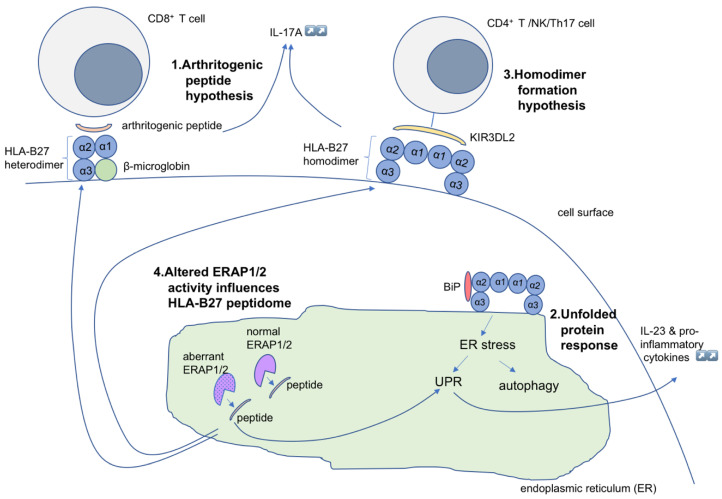
Figure 3.
Illustration of the hypothesis for the pathogenetic role of HLA-B*2705 molecules in spondyloarthritis. (1) Arthritogenic peptides displayed by properly folded HLA-B*27 can be recognized by autoreactive CD8+ T cells, resulting in inflammation. (2) Misfolded HLA-B*27 chains and binding of BiP causes ER stress and activation of UPR, leading to increased production of IL-23 and other proinflammatory cytokines. (3) Cell surface HLA homodimers interact with CD4+ T cells through innate immune receptors, such as KIR3DL2, and facilitate cell-mediated autoimmune responses. (4) Altered ERAP1 activity can result in changes in peptide processing, with pathological consequences. ER, endoplasmic reticulum; ERAP1, ER aminopeptidases (ERAP)1; KIR3DL2, killer immunoglobulin-like receptor; UPR, unfolded protein response.