Abstract
Hydrogen sulfide (H2S) together with polysulfides (H2Sn, n > 2) are signalling molecules like NO with various physiological roles including regulation of neuronal transmission, vascular tone, inflammation and oxygen sensing. H2S and H2Sn diffuse to the target proteins for S‐sulfurating their cysteine residues that induces the conformational changes to alter the activity. On the other hand, 3‐mercaptopyruvate sulfurtransferase transfers sulfur from a substrate 3‐mercaptopyruvate to the cysteine residues of acceptor proteins. A similar mechanism has also been identified in S‐nitrosylation. S‐sulfuration and S‐nitrosylation by enzymes proceed only inside the cell, while reactions induced by H2S, H2Sn and NO even extend to the surrounding cells. Disturbance of signalling by these molecules as well as S‐sulfuration and S‐nitrosylation causes many nervous system diseases. This review focuses on the signalling by H2S and H2Sn with S‐sulfuration comparing to that of NO with S‐nitrosylation and discusses on their roles in physiology and pathophysiology.
Keywords: 3‐mercaptopyruvate sulfurtransferase, hydrogen sulfide, nitric oxide, polysulfides, S‐nitrosylation, S‐sulfuration
Abbreviations
- 3MP
3‐mercaptopyruvate
- ADAM17
disintegrin and metalloproteinase domain‐containing protein 17
- Aβ1–42
amyloid β‐protein
- BACE
β‐secretase
- CAT
cysteine aminotransferase
- CBS
cystathionine β‐synthase
- CFTR
cystic fibrosis transmembrane conductance receptor
- CSE
cystathionine γ‐lyase
- EE
ethylmalonyl encephalopathy
- ETHE1
sulfur dioxygenase
- FADH2
flavin adenine dinucleotide
- GCL
glutamate cysteine ligase
- H2Sn
hydrogen polysulfides
- HSNO
thionitrous acid
- HSSNO
nitrosopersulfide
- Keap1
Kelch‐like ECH‐associated protein 1
- MPST
3‐mercaptopyruvate sulfurtransferase
- Nrf2
nuclear factor erythroid 2‐related factor 2
- TRPA1
transient receptor potential ankyrin 1
- γ‐GCS
γ‐glutamyl cysteine synthetase
1. INTRODUCTION
A diffusing factor, which is released from vascular endothelium by a stimulation with ACh to relax vascular smooth muscle, was discovered and called endothelium‐derived relaxation factor (EDRF) (Furchgott & Zawadzki, 1980). It was later identified as NO (Ignarro, Buga, Wood, Byrns, & Chaudhuri, 1987; Palmer, Ferrige, & Moncada, 1987), which activates soluble guanylate cyclase and subsequently PKG through cGMP (Arnold, Mittal, Katsuki, & Murad, 1977). In the brain, an excitatory neurotransmitter glutamate had been recognized to induce cGMP (Mao, Guidotti, & Costa, 1974) and the activation of NMDA receptors by glutamate was found to induce a release of a diffusible factor which had similar properties to EDRF in a Ca2+‐dependent manner (Garthwaite, Charles, & Chess Williams, 1988). NO producing activity was identified as an enzymatic reaction with arginine as a substrate in NADPH and Ca2+/calmodulin‐dependent manner (Bredt & Snyder, 1990). The activity is localized to neurons (neuronal NOS [nNOS]), vascular endothelial cells (endothelial NOS [eNOS]) and the inducible activity (inducible NOS [iNOS]) (Bredt et al., 1991).
Hydrogen sulfide (H2S), which was identified in the brain (Goodwin et al., 1989; Savage & Gould, 1990; Warenycia, Goodwin, et al., 1989), has physiological roles including cognitive function (Abe & Kimura, 1996) and vascular tone regulation (Hosoki, Matsuki, & Kimura, 1997; Zhao, Zhang, Lu, & Wang, 2001). H2S is produced by four enzymes cystathionine β‐synthase (CBS), cystathionine γ‐lyase (CSE), 3‐mercaptopyruvate sulfurtransferase (MPST) and l‐cysteine:2‐oxoglutarate aminotransferase (CAT) or d‐amino acid oxidase, from d/l‐cysteine as a source of sulfur (Abe & Kimura, 1996; Chiku et al., 2009; Hosoki et al., 1997; Mikami, Shibuya, Kimura, Ogasawara, & Kimura, 2011; Nagahara, Yoshii, Abe, & Matsumura, 2007; Shibuya et al., 2009; Shibuya et al., 2013; Stipanuk & Beck, 1982). CBS is localized to astrocytes in the brain but most intensively in cerebellar Bergmann glia (Enokido et al., 2005), while the levels of CSE and its activity are significantly low in the brain (Ishii et al., 2004). MPST is localized to both neurons and astrocytes in the brain (Nagahara, Ito, Kitamura, & Nishino, 1998; Shibuya et al., 2009). DAO is expressed in the brain stem, cerebellum and spinal cord, exclusively astrocytes including Bergmann glia (Horiike, Tojo, Arai, Nozaki, & Maeda, 1994). CAT, which is identical to aspartate aminotransferase, is expressed mainly in the cerebellum (Lin & Chen, 1983).
H2S induces Ca2+ influx in astrocytes by activating transient receptor potential ankyrin 1 (TRPA1) channels and we later found that hydrogen polysulfides (H2Sn, n > 2) activate the channels more effectively than H2S does (Kimura et al., 2013; Nagai, Tsugane, Oka, & Kimura, 2004, 2006; Oosumi et al., 2010). H2S2 and H2S3 were identified in the brain and MPST produces H2Sn, and other persulfurated molecules such as cysteine persulfide, GSH persulfide and persulfurated proteins (Kimura et al., 2017; Kimura et al., 2015; Koike et al., 2017; Nagahara, 2018; Nagahara, Koike, Nirasawa, Kimura, & Ogasawara, 2018; see also Kimura, 2020). H2Sn are oxidized by sulfur dioxygenase to sulfite (H2SO3), which is further oxidized by rhodanese to thiosulfate (H2S2O3).
Two modes of action have been proposed for both H2S and NO signalling. One is that both molecules diffuse to the haem for redox reaction (Ruetz et al., 2017; Vitvitsky, Yadav, Kurthen, & Banerjee, 2015) as well as to cysteine residues of the target proteins to S‐sulfurate, covalently react sulfur with a thiol of cysteine residues (S‐sulfhydrate, persulfidate; see Toohey, 2012) or S‐nitrosylate, covalently react NO with a critical protein thiol, them (Lancaster, 2017; Mustafa et al., 2009). The other is that sulfur and NO are transferred from respective enzymes to the target proteins for S‐sulfuration and S‐nitrosylation. An example of the first mode of action of NO is that of EDRF, which diffuses from endothelium to vascular smooth muscle (Furchgott & Zawadzki, 1980). In the nervous system, NO diffuses from postsynapse to presynapse as a retrograde transmitter to induce a release of neurotransmitter glutamate (Garthwaite, 1991; Garthwaite et al., 1988; Zhuo, Small, Kandel, & Hawkins, 1993). On the other hand, the enzyme‐mediated S‐nitrosylation proceeds by clusters of enzymes which generate NO, synthesize S‐nitrosylated proteins, and transnitrosylate it that is the reaction to transfer of an NO group from one protein to another (Nakamura & Lipton, 2013; Seth et al., 2018).
H2S diffuses to haem of target proteins to regulate their activity or to the target cysteine disulfide to reduce it (Abe & Kimura, 1996; Aizenman, Lipton, & Loring, 1989; Matsui et al., 2018). H2S and H2Sn diffuse to the targets to S‐sulfurate cysteine residues to modify their activity (Kimura et al., 2015; Mustafa et al., 2009). As to enzyme‐mediated S‐sulfuration, MPST transfers sulfur from 3‐mercaptopyruvate (3MP) to cysteine residues of target proteins (Kimura et al., 2017; Nagahara, Nirasawa, Yoshii, & Niimura, 2012).
H2S exerts regulatory, beneficial, and protective effects at physiological concentrations, while it is toxic at higher concentrations. In Down's syndrome and ethylmalonyl encephalopathy, the levels of H2S and/or H2Sn are increased and cause damage to the brain (Panagaki, Randi, Augsburger, & Szabo, 2019; Tiranti et al., 2009). For Down's syndrome and ethylmalonyl encephalopathy, a decrease in the levels of H2S has been proposed to have therapeutic potential. In contrast, in Huntington's disease and Alzheimer's disease, H2S levels are not enough to properly function (Cao et al., 2018; Paul et al., 2014; Sbodio, Snyder, & Paul, 2016, 2018; Vandini et al., 2019). For these diseases, a supplementation of H2S may have a benefit. In schizophrenia, both beneficial and toxic effects of H2S and H2Sn have been reported (Ide et al., 2019; Topcuoglu et al., 2017; Ünal, Erzin, Yüksel, Alisik, & Erel, 2018; Xiong et al., 2018). The balance of H2S, H2Sn and NO as well as S‐sulfuration together with S‐nitrosylation plays an important role for the pathogenesis of these neuronal diseases.
2. S‐SULFURATION BY H2S, H2Sn AND MPST
Mustafa et al. (2009) have demonstrated that S‐sulfuration of cysteine residues of target proteins as a mode of action of H2S. GAPDH is activated through S‐sulfuration by H2S (Mustafa et al., 2009). It was later reported that the activity of GAPDH is suppressed by S‐sulfuration by H2Sn rather than H2S (Jarosz et al., 2015). S‐sulfuration depends on the redox condition of the target cysteine residues; H2S S‐sulfurates oxidized cysteine like Cys‐SOH or Cys‐SNO, while H2Sn S‐sulfurate cysteine (Cys‐SH) (Figure 1) (Mishanina, Libiad, & Banerjee, 2015). The activity of GAPDH depends on the redox state of its active cysteine residues.
FIGURE 1.
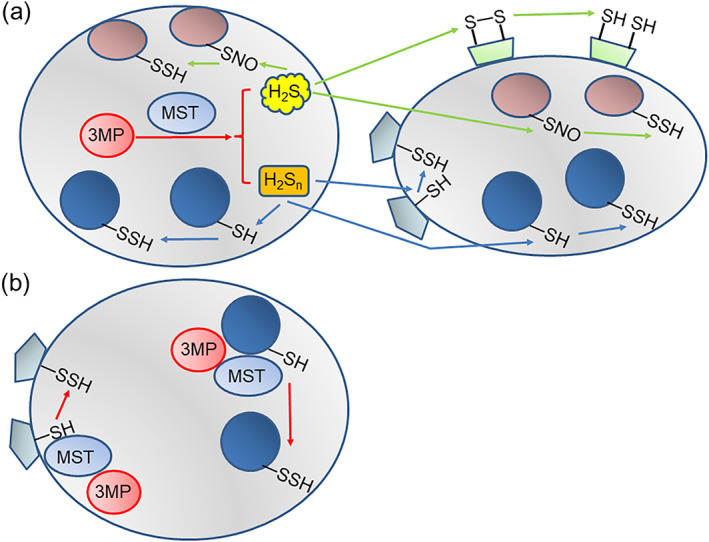
H2Sn can transmit signals across cells, while S‐sulfuration by 3‐mercaptopyruvate sulfurtransferase (MPST) is restricted only inside the cells. (a) H2S and H2Sn can pass through plasma membrane of the cell in which they are produced and reach the nearby cells as well as react with the targets inside the produced cell. H2S S‐sulfurates the S‐nitrosylated cysteine residues, while H2Sn S‐sulfurate the cysteine residues. (b) MPST transfers sulfur from 3MP to targets inside the cell
H2Sn activate TRPA1 channels which have two sensitive cysteine residues at the amino terminus, suggesting that the two residues are S‐sulfurated or one of them is S‐sulfurated to react with the other to make cysteine disulfide bridge (Hatakeyama, Takahashi, Tominaga, Kimura, & Ohta, 2015; Kimura, 2015a; Kimura et al., 2013; Nagai et al., 2004, 2006; Oosumi et al., 2010; Streng et al., 2008). A similar mechanism was reported for the regulation of tumour suppressor phosphatase and tensin homologue in which one cysteine residue is S‐sulfurated by H2Sn and then reacts with another non‐S‐sulfurated one to produce a cysteine disulfide bond, leading to the conformational change (Greiner et al., 2013). Protein kinase G‐1α (PKG1α) is inactive at its monomer, while it is activated by forming dimer which is generated by S‐sulfuration of one cysteine residue of a monomer that reacts with a counterpart cysteine residue of another monomer to produce cysteine disulfide bridge between the two (Kimura, 2020; Stubbert et al., 2014).
MPST associated with thioredoxin produces H2S and H2Sn as well as other persulfurated molecules (Figure 2) (Kimura et al., 2017; Kimura et al., 2015; Mikami et al., 2011). The endogenous levels of H2S and H2S2 in the brain are 0.030 ± 0.004 μmol·g−1 protein (approximately 3.0 μM) and 0.026 ± 0.002 μmol·g−1 protein (2.6 μM), respectively (Koike et al., 2017). Because H2S2 and H2S3 efficiently S‐sulfurate cysteine and GSH to produce cysteine persulfide and GSH persulfide (Kimura et al., 2017), once H2Sn are produced, cysteine and GSH exist nearby can immediately be S‐sulfurated. Since no enzyme has been identified to mediate the reaction of H2S with haem containing proteins such as Hb, neuroglobin and catalase as well as sulfur quinone oxidoreductase and cupper/zinc SOD (Olson et al., 2018; Olson et al., 2017; Ruetz et al., 2017; Searcy, 1996; Searcy, Whitehead, & Maroney, 1995; Vitvitsky et al., 2015), H2S must reach these targets by diffusion.
FIGURE 2.
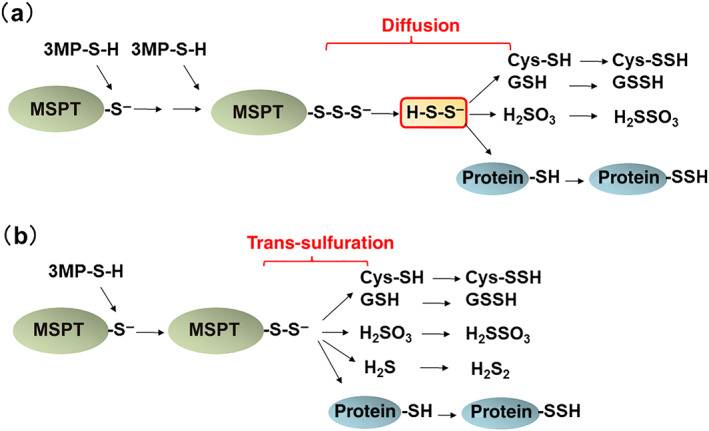
S‐sulfuration by H2Sn diffusion and that by 3‐mercaptopyruvate sulfurtransferase (MPST). (a) MPST produces H2Sn which reach by diffusion to S‐sulfurate H2S, cysteine, GSH, and cysteine residues of proteins. (b) MPST transfers sulfur from 3‐mercaptopyruvate (MP) to H2S, cysteine, GSH, and cysteine residues of proteins without being mediated by H2Sn (trans‐sulfuration). This figure is produced by modifying Kimura et al. (2017)
How do H2Sn, oxidized molecules of H2S, survive in the relatively reducing atmosphere of the cell? In the intracellular milieu, all the cysteine residues are not necessarily thiol, but some of them are oxidized. Under basal conditions, 10% to 25% of proteins are S‐sulfurated (R‐SSH) with a few % S‐nitrosylated (R‐SNO), and remaining 70% to 90% are proteins with thiol (R‐SH) (Jaffrey, Erdjument‐Bromage, Ferris, Tempst, & Snyder, 2001; Mustafa et al., 2009). It is also supported by our observations that brain homogenates absorb H2S as bound sulfane sulfur (S‐sulfurated molecules including proteins), which releases H2S under reducing conditions such as in the presence of dithiothreitol (DTT) (Ishigami et al., 2009), suggesting that cells contain oxidized cysteine residues such as S‐nitrosylated ones, which can react with H2S to produce S‐sulfurated cysteine residues or bound sulfane sulfur. H2S is released from bound sulfane sulfur in brain homogenates (without the pre‐absorption of H2S) in the presence of DTT (1,481 + 174 nmol·g−1 protein of brain homogenates at pH 7.4) (Ishigami et al., 2009). These observations suggest that S‐sulfurated and S‐nitrosylated proteins exist together with proteins with thiol in cells. The ratio of S‐sulfurated, S‐nitrosylated proteins to intact cysteine residues of proteins must well be balanced with that of corresponding free molecules in cells (Figure 3).
FIGURE 3.
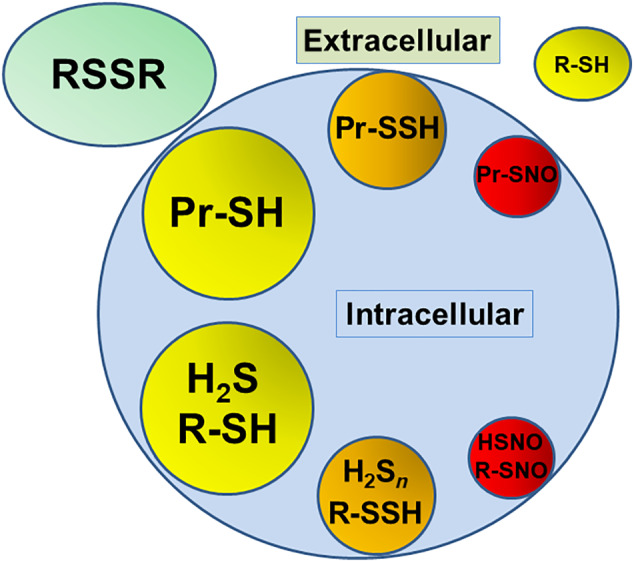
The ratio of S‐sulfurated, S‐nitrosylated proteins to intact cysteine residues of proteins must well be balanced with that of corresponding free molecules in cells. In the extracellular milieu, oxidized glutathione (GSH) and cystine are dominant with much less amount of cysteine. Pr, protein; R, cysteine and GSH
MPST transfers sulfur from 3MP to cysteine as observed in the following. (1) MPST can produce H2S, H2Sn, cysteine persulfide, and GSH persulfide by transferring sulfur to cysteine (Kimura et al., 2017; Kimura et al., 2015). (2) The levels of persulfurated molecules, which were measured as H2S released in the presence of a reducing agent DTT called as bound sulfane sulfur (Ishigami et al., 2009; Kimura, 2015a; Ogasawara, Ishii, Togawa, & Tanabe, 1993; Ogasawara, Isoda, & Tanabe, 1994; Warenycia et al., 1990), were greater in cells expressing MPST compared to a control (Shibuya et al., 2009). (3) Administration of a substrate cysteine to mice increased the amounts of bound sulfane sulfur in tissues expressing MPST, while there was no change in those administered saline (Shibuya et al., 2013). (4) The levels of bound sulfane sulfur were less than half in the brains of MPST knockout mice than those in the wild‐type mice (Kimura et al., 2017). S‐sulfuration by MPST is specific but restricted inside cells, while that by diffusion extends the signalling to nearby cells.
3. S‐NITROSYLATION AND THE INTERACTION BETWEEN H2S AND NO
For S‐nitrosylation, two mechanisms have been proposed. NO was initially thought only to diffuse after being produced by NOS to the target proteins to S‐nitrosylate them (Lancaster, 2017). However, it has been demonstrated that S‐nitrosylation proceeds by clusters of enzymes which generate NO, synthesize S‐nitrosylated proteins, and transnitrosylate it (Seth et al., 2018). Signalling by H2S, H2Sn and NO as well as S‐sulfuration and S‐nitrosylation must have both diffusion‐mediated and enzyme‐oriented mechanisms for regulating the activity of targets.
Synergistic effect of H2S with NO on vascular relaxation led to the identification of two mechanisms (Hosoki et al., 1997). One is that the chemical interaction between H2S and NO produces H2Sn, nitrosothiol, thionitrous acid (HSNO), and nitrosopersulfide (HSSNO) that have the relaxation effect greater than each parental molecule (Cortese‐Krott et al., 2015; Eberhardt et al., 2014; Filipovic et al., 2012; Miyamoto et al., 2017; Moustafa & Habara, 2016; Nagai et al., 2006; Oosumi et al., 2010; Stubbert et al., 2014; Whiteman et al., 2006; see also Kimura, 2020). Another mechanism is that H2S and NO mutually regulate their synthesizing enzymes (Kimura, 2016; King et al., 2014; Kondo et al., 2013; Minamishima et al., 2009; Zhao et al., 2001). For chemical interaction between H2S and NO, both molecules should diffuse to interact with each other.
After the identification of EDRF as NO, there appeared a discrepancy. EDRF hyperpolarizes the membrane of vascular smooth muscle, while NO has little hyperpolarizing effect. Based on this observation, it has been suggested that EDRF contains an additional factor, endothelium‐derived hyperpolarizing factor (EDHF) (Chen, Suzuki, & Weston, 1988). Because H2S activates KATP channels and hyperpolarizes the membrane potential, it has been proposed as a potential EDHF (Hosoki et al., 1997; Mustafa et al., 2011; Zhao et al., 2001). Another possibility is H2Sn, which are produced by the chemical interaction of H2S with NO and activate PKG1α to relax vasculature and are also potent substances to activate KATP channels by S‐sulfurating the active cysteine residue (Cortese‐Krott et al., 2015; Eberhardt et al., 2014; Filipovic et al., 2012; Miyamoto et al., 2017; Moustafa & Habara, 2016; Mustafa et al., 2011; Nagai et al., 2006; Oosumi et al., 2010; Stubbert et al., 2014; Whiteman et al., 2006; see also Kimura, 2020).
4. PHYSIOLOGICAL ROLES OF H2S, H2Sn, AND NO
4.1. Neuronal plasticity
When a neurotransmitter glutamate activates postsynaptic NMDA receptors, Ca2+ influx is induced and Ca2+/calmodulin‐dependent nNOS is subsequently activated to produce NO (Garthwaite et al., 1988). NO produced at post‐synaptically crosses synaptic cleft to pre‐synaptic terminal as a retrograde transmitter, which modifies a release of neurotransmitter glutamate, leading to the facilitation of hippocampal LTP (O'Dell, Hawkins, Kandel, & Arancio, 1991). NO also modifies LTP formation to alter the activity post‐synaptically (Taqatqeh et al., 2009).
H2S enhances the activity of NMDA receptors at the active synapses by reducing the cysteine disulfide bond located at the hinge of a ligand binding domain of the receptors to facilitate LTP induction (Abe & Kimura, 1996; Aizenman et al., 1989). On the other hand, H2Sn activate TRPA1 channels in astrocytes surrounding the synapse to induce Ca2+ influx, which triggers a release of a gliotransmitter d‐serine to the synaptic cleft to enhance the activity of NMDA receptors (Kimura et al., 2013; Nagai et al., 2004, 2006; Oosumi et al., 2010; Shigetomi, Jackson‐Weaver, Huckstepp, O'Dell, & Khakh, 2013). Genetic knockdown of CBS impairs LTP, while S‐sulfuration of serine racemase, which generates d‐serine from l‐serine, restores LTP (Li et al., 2017).
4.2. Neuroprotective role
There are two forms of glutamate toxicity; excitotoxicity is caused by the immoderate excitation of NMDA receptors which transport excessive Ca2+ into neurons to death (Choi, 1988). In oxidative glutamate toxicity called oxytosis, which is quite similar to ferroptosis (Lewerenz, Ates, Methner, Conrad, & Maher, 2018), high concentrations of glutamate suppress cystine/glutamate antiporter, leading to the decreased transport of cystine into cells that causes decreased production of GSH, an intracellular major antioxidant, resulting in making cells vulnerable to oxidative stress (Murphy, Miyamoto, Sastre, Schnaar, & Coyle, 1989; Tan, Schubert, & Maher, 2001). H2S protects embryonic neurons from oxidative glutamate toxicity (Kimura & Kimura, 2004). H2S enhances the activity of cystine/glutamate antiporter xCT to transport cystine, which is reduced to cysteine to be used for the production of GSH. H2S also augments the activity of glutamate cysteine ligase (GCL), a rate limiting enzyme for GSH production, also known as γ‐glutamyl cysteine synthetase (γ‐GCS) (Kimura, Goto, & Kimura, 2010; Kimura & Kimura, 2004). The activity of ATP‐dependent K+ channels and cystic fibrosis transmembrane conductance receptor (CFTR) Cl− channels is also activated by H2S to suppress the excess excitation of the neurons (Kimura, Dargusch, Schubert, & Kimura, 2006). H2Sn S‐sulfurate Kelch‐like ECH‐associated protein 1 (Keap1) to release nuclear factor erythroid 2‐related factor 2 (Nrf2) from Keap1/Nrf2 complex to nucleus where Nrf2 up‐regulates antioxidant genes, leading to the protection of neurons from oxidative stress.
H2S and H2Sn are further oxidized to thiosulfate, sulfite and sulfate. Sulfite protects embryonic neurons from oxidative stress as efficiently as H2S and H2Sn, while thiosulfate and sulfate do not (Kimura, Shibuya, & Kimura, 2019). It reacts with cystine to produce cysteine, which is more efficiently transported into cells than cystine, leading to the effective production of GSH (Clarke, 1932). A counterpart product of this reaction S‐cysteine sulfonate is agonist of NMDA receptors (Clarke, 1932; Kumar et al., 2018). Because matured neurons express NMDA receptors, of which the activity is enhanced by H2S and S‐cysteinesulfonate (Abe & Kimura, 1996; Choi, 1988; Kumar et al., 2018; Murphy et al., 1989; Tan et al., 2001), the simultaneous application of inhibitors for NMDA receptors must be required for the protection of matured neurons.
Nagahara et al. have proposed that S‐sulfuration has a role to protect proteins from oxidative stress. In subsequent oxidation of cysteine–SH to –SOH, –SO2H, and –SO3H, only –SOH is reversible to –SH, but further oxidized forms are irreversible. In contrast, the oxidation of per‐sulfurated cysteine–SSO2H and –SSO3H can be converted to cysteine–SH by the reduced form of thioredoxin (Nagahara et al., 2012). These observations were reproduced and confirmed by other groups (Dóka et al., 2020; Zivanovic et al., 2019). The levels of S‐sulfuration are declined associated with aging that is a risk factor for the neurodegenerative diseases (Zivanovic et al., 2019).
NO protects neurons from excitotoxicity by decreasing the excessive influx of Ca2+ by suppressing the activity of NMDA receptors through S‐nitrosylation (Choi et al., 2000; Jaffrey et al., 2001). NO also exerts cytoprotective effects by suppressing caspase activity through S‐nitrosylation of its active site cysteine (Melino et al., 1997). Because the suppression of soluble guanylate cyclase showed a similar cell death effect to that by the deprivation of NO, cGMP may be involved in the cytoprotective effect of NO (Contestabile & Ciani, 2004).
4.3. Mitochondrial energy formation
H2S is well‐known toxic gas at high concentrations and its toxicity is attributed to the inhibition of mitochondrial cytochrome c oxidase by suppressing the binding of oxygen (Hill et al., 1984), though its effect is not so potent compared to that of azide (Umemura & Kimura, 2007). There has been the hypothesis that mitochondria originated from sulfide‐oxidizing symbionts. Yong and Searcy (2001) demonstrated that chicken liver mitochondria consumed oxygen at an accelerated rate when supplied with low concentrations of H2S, and H2S oxidation is coupled to ATP generation. ATP synthesis requires less than 5‐μM H2S and maximum respiration is induced at 10 μM and less efficient up to 60 μM (Yong & Searcy, 2001).
The balance between H2S as the electron donor and the inhibitor of cytochrome c oxidase is likely controlled by H2S and oxygen availability. Low concentrations of H2S preserve the respiratory rate, while high concentrations inhibit it (Abou‐Hamdan et al., 2016). In contrast to NO and carbon monoxide, H2S binds to sulfur quinone oxidoreductase that transfers two electrons and two protons from H2S to coenzyme Q. A pair of electrons ultimately reduces a single oxygen atom at cytochrome c oxidase (Figure 4) (Goubern, Andriamihaja, Nübel, Blachier, & Bouillaud, 2007). Because sulfide oxidation requires three times more oxygen than that of NADH or flavin adenine dinucleotide (FADH2), sulfide may be a poor energy substrate (Lagoutte et al., 2010). However, Szabo et al. suggested that this low energy yield is balanced by unique properties of H2S. (1) H2S freely diffuses across membrane without the need of transporters and (2) the affinity of H2S to sulfide oxidation unit including sulfur quinone oxidoreductase is high and 100% is oxidized (Szabo et al., 2014). In the brain, the expression of sulfur quinone oxidoreductase is very low (Linden et al., 2012); it is predicted that a haemoprotein neuroglobin, which is primarily expressed in neurons, plays a role in H2S oxidation (Ruetz et al., 2017).
FIGURE 4.
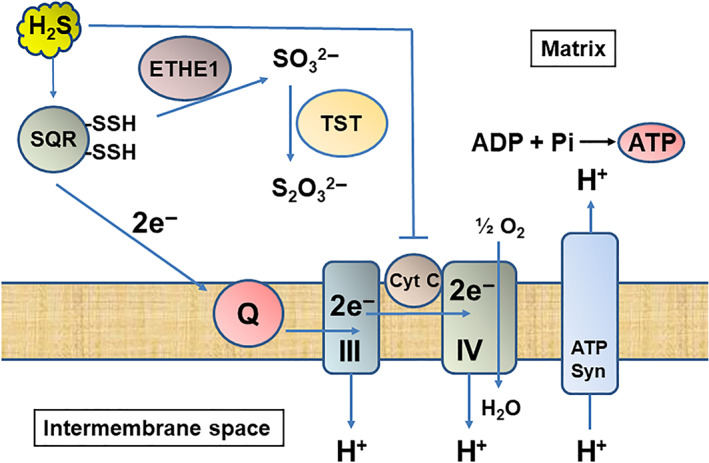
H2S is used for ATP production at physiological concentrations, while it inhibits cytochrome c oxidase at higher concentrations in mitochondria. H2S is metabolized by sulfur quinone oxidoreductase (SQR), sulfur dioxygenase (ETHE1, and rhodanese (TST) to thiosulfate through sulfite. Electrons are sent to coenzyme Q to complex IV through III and used for pumping out H+ from matrix to intermembrane space. ATP synthase produces ATP using the gradient of H+. In contrast, high concentrations of H2S suppress cytochrome c oxidase and the energy formation
5. PATHOPHYSIOLOGICAL ROLE OF H2S, H2Sn AND NO
The overproduction of H2S suppresses cytochrome c oxidase that is likely to be involved in the pathogenesis of Down's syndrome and ethylmalonic encephalopathy (Fernandez Cardoso et al., 2017; Marechal et al., 2019; Panagaki et al., 2019; Tiranti et al., 2009). In contrast, the lack of H2S production may cause the pathology of Parkinson's disease, Huntington's disease and Alzheimer's disease (Cao et al., 2018; Chung et al., 2004; He et al., 2016; Paul et al., 2014; Sbodio et al., 2016, 2018; Vandiver et al., 2013; Wright et al., 2016; Xie et al., 2013). The detrimental effects caused by the excess production of H2S and H2Sn (Ide et al., 2019) as well as those by lacking in the neuroprotective effect of these molecules (Topcuoglu et al., 2017; Ünal et al., 2018; Xiong et al., 2018) have been reported for the pathogenesis of schizophrenia.
6. TOXICITY OF H2S AND H2Sn
6.1. Down's syndrome
Down's syndrome is characterized by impaired brain growth and maturation, which causes mental retardation, and has a trisomy of chromosome 21. CBS is encoded on chromosome 21 (21q22.3), and the expression of CBS mRNA is 12 times greater in myeloblasts of Down's syndrome children than those of normal individuals (Taub et al., 1999). We found that CBS protein levels in Down's syndrome brains are approximately 3 times greater than those in the normal brains that is twice greater than those expected from the trisomy (Ichinohe et al., 2005). In addition, elderly adults of Down's syndrome are associated with an Alzheimer's type of dementia where CBS is localized to astrocytes that surround senile plaques in the brain (Ichinohe et al., 2005).
The greater levels of thiosulfate, a metabolite of H2S, were detected in urine of DS patients compared to that of normal individuals (Kamoun, Belardinelli, Chabli, Lallouchi, & Chadefaux‐Vekemans, 2003). The increased production of H2S by overexpressed CBS may be the cause of the neurological impairments in Down's syndrome patients (Figure 5a). In a mouse model of Down's syndrome, three copies of CBS gene are necessary to cause the Down's syndrome‐related recognition memory deficit (Marechal et al., 2019). Panagaki et al. (2019) showed that the levels of CBS and H2S are markedly elevated in Down's syndrome fibroblast cells and the mitochondrial electron transport, oxygen consumption, and ATP generation are profoundly suppressed. They suggested the therapeutic potential of CBS inhibitors.
FIGURE 5.
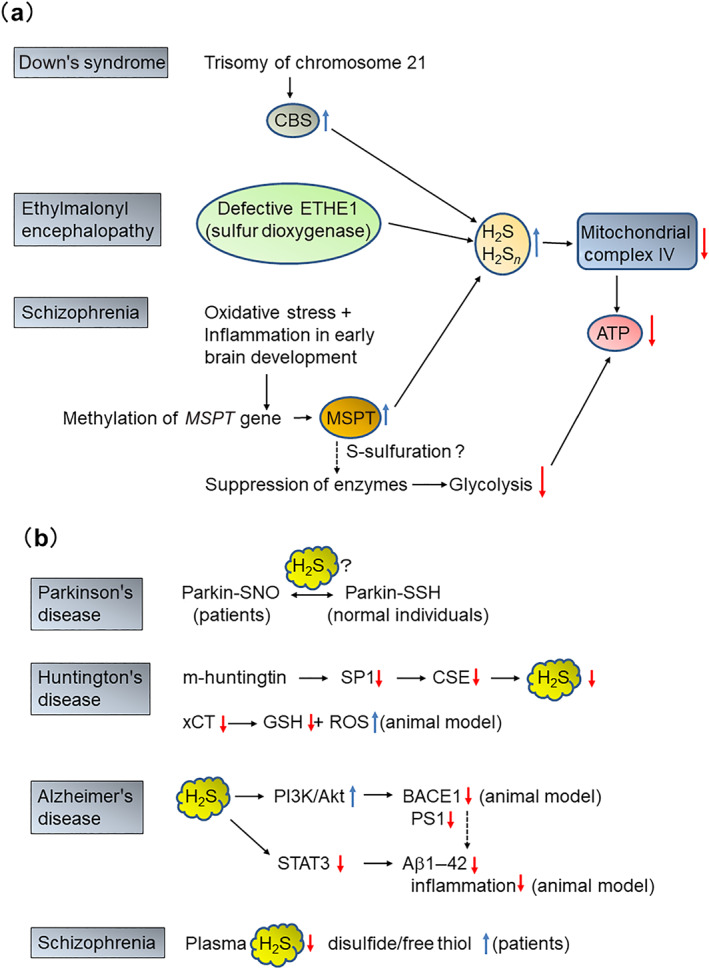
Diseases caused by unbalance of H2S and H2Sn in the CNS. (a) Diseases caused by an excess amount of H2S and H2Sn. A trisomy of chromosome 21, on which cystathionine β‐synthase (CBS) is encoded, increases the levels of CBS in Down's syndrome. Sulfur dioxygenase, which is encoded in sulfur dioxygenase gene (ETHE1) and one of the enzymes metabolizing H2S and H2Sn, is defective in ethylmalonyl encephalopathy. Methylation of 3‐mercaptopyruvate sulfurtransferase (MPST) gene increases the production of MPST in schizophrenia. Glycolysis is decreased by the suppression of several enzymes such as triosephosphate isomerase, phosphoglycerate kinase, and phosphopyruvate hydratase. In these diseases, cytochrome c oxidase is suppressed by high concentrations of H2S, resulting in the decreased production of ATP. (b) Diseases caused by a lack of H2S or H2Sn in the CNS. S‐sulfurated parkin in normal individuals is active, while parkin with the same cysteine residues being S‐nitrosylated in Parkinson's disease (PD) brains is inactive. Mutant huntingtin suppresses the transcription of cystathionine γ‐lyase (CTL), CTH gene, from specificity protein 1 (SP1), resulting in the decrease in the production of H2S. In Huntingdon's disease (HD) animal model, the activity of cystine/glutamate antiporter xCT is suppressed, and GSH levels are decreased, while those of ROS increased. The animal model of Alzheimer's disease (AD) shows that H2S suppresses PI3K/Akt, while enhances the activity of STAT3. Both effects result in the decreased production of Aβ1–42 and suppression of inflammation. In some report, the plasma levels of H2S are lower in patients of schizophrenia than normal individuals
6.2. Ethylmalonyl encephalopathy
Ethylmalonyl encephalopathy is an autosomal recessive early onset and defective in cytochrome c oxidase in muscle and brain and excretes ethylmalonic acid in urine. In this disease, ETHE1, a gene encoding a β‐lactamase‐like iron‐coordinating metalloprotein or sulfur dioxygenase, is deficient. A great amount of thiosulfate, a metabolite of H2S, is excreted in urine of Ethe1 knockout mice and patients with this disease (Tiranti et al., 2009). H2S is mainly metabolized by mitochondrial enzymes, sulfur quinone oxidoreductase, sulfur dioxygenase, and rhodanese. In the brain and skeletal muslce deficiency of sulfur dioxygenase (ETHE1) inceases the basal levels of H2S which suppress the cytochrome c oxidase that may lead to progressive neurological failure (Tiranti et al., 2009) (Figure 5a). In addition, acyl‐protein thioesterase, which hydrolyses fatty acids bound to cysteine and GSH transferases are suppressed in Ethe1 knockout mice probably due to the increased levels of H2S or persulfides (Hildebrandt, Meo, Zeviani, Viscomi, & Braun, 2013).
The administration of metronidazole, an antibiotic, or N‐acetylcysteine prolonged the lifespan of Ethe1 knockout mice and marked clinical improvement in patients with ethylmalonyl encephalopathy (Viscomi et al., 2010). Metronidazole may be expected to decrease the levels of H2S incorporated into blood from intestinal bacteria. N‐acetylcysteine is metabolized in cells to cysteine, which is a precursor of GSH being predicted to buffer H2S, while it is also a substrate to produce H2S. In this instance, the former may be a dominant mechanism of N‐acetylcysteine for the improvement of the disease.
H2S decreased the activities of citrate synthase, aconitase, and creatine kinase in the brain of mouse model of ethylmalonyl encephalopathy (Fernandez Cardoso et al., 2017). H2S also suppressed mitochondrial respiration, decreased mitochondrial membrane potential and induced swelling caused by calcium in brain mitochondria. Changes in mitochondrial membrane potential and the swelling caused by H2S may be due to opening of mitochondrial permeability transition pore (Fernandez Cardoso et al., 2017). Bioenergetics disturbance, lipid peroxidation and mitochondrial permeability transition pore opening mediated by H2S may be involved in the pathophysiology of brain damage observed in this disease (Fernandez Cardoso et al., 2017).
7. H2S AND H2Sn ARE BENEFICIAL
7.1. Parkinson's disease
Parkinson's diseas is a neurodegenerative disorder mainly causing motor dysfunction. Parkin and α‐synuclein are associated with pathophysiology of Parkinson's disease. α‐Synuclein is a major component of Lewy bodies associated with rare cases of Parkinson's disease, while parkin, which is an E3 ubiquitin ligase that ubiquitinates diverse substrates, is responsible for the clearance of misfolded proteins including α‐synuclein and its mutations cause autosomal recessive Parkinson's disease (Choi et al., 2001; Jęśko, Lenkiewicz, Wilkaniec, & Adamczyk, 2019). The activity of parkin is suppressed by S‐nitrosylation and thereby decreases its protective role in the brains of Parkinson's disease patients (Chung et al., 2004). In contrast, in the brains of normal individuals, it is active and S‐sulfurated. Cys95, Cys59, and Cys182 are S‐sulfurated in normal individuals, while the same cysteine residues are S‐nitrosylated in Parkinson's disease brains (Vandiver et al., 2013) (Figure 5b). Parkin is an example of complementary roles of the S‐sulfuration and S‐nitrosylation. H2S may be involved in the conversion of S‐nitrosylated cysteine to S‐sulfurated one in parkin to improve its function, while NO may S‐nitrosylate cysteine residues to decrease the protective effect of parkin (Chung et al., 2004; Vandiver et al., 2013; Yao et al., 2004).
Systemic administration of sodium salt of H2S, NaHS, dramatically reversed the progression of movement dysfunction and loss of dopaminergic neurons in the Parkinson's disease model rats (Hu et al., 2010). A similar effect was observed with an H2S‐releasing l‐DOPA derivative compound (Xie et al., 2013). Overexpression of CBS increased the endogenous levels of H2S, reversed the behaviour induced in Parkinson's disease model rats, and decreased apoptotic neuronal loss of the nigral dopaminergic neurons (Xie et al., 2013). The application of H2S and the regulation of CBS activity have a therapeutic potential (Yin, Yin, Huang, & Wu, 2017).
7.2. Huntington's disease
Huntington's disease is a devastating neurodegenerative disorder characterized by the progressive development of involuntary movements, neuropsychiatric symptoms, and cognitive impairment (Cepeda & Tong, 2018). Huntington's disease belongs to triplet repeat diseases, in which elongated polyglutamine stretches affect the protein product, and the mutant huntingtin disrupts a number of vital cellular processes, including neuronal transmission, metabolism, and gene transcription (Chaganti, McCusker, & Loy, 2017).
Although the levels of CSE are very low in the brain compared to other tissues (Ishii et al., 2004), those are even much lower in mouse models of Huntington's disease and human Huntington's disease brain compared to a control mice and normal individuals, respectively (Paul et al., 2014). It is caused by the suppression of the transcription factor specificity protein 1, which regulates the transcription of CSE, by mutant huntingtin (Ishii et al., 2004; Paul et al., 2014) (Figure 5b). The expression of CSE is also regulated by transcription factor 4 (ATF4), which is dysfunctional in Huntington's disease, under endoplasmic reticulum (ER) stress or amino acid deficiency (Sbodio et al., 2016). Striatal cell lines derived from Huntington's disease model mice have decreased levels of cystine/glutamate antiporter xCT mRNA and protein expression, leading to the lower basal levels of GSH and higher basal levels of ROS (Wright et al., 2016) (Figure 5b). Administration of N‐acetylcysteine ameliorates Huntington's disease pathology in this model.
7.3. Alzheimer's disease
Alzheimer's disease is a chronic neurodegenerative disease that affects cognitive functions and memory formation. The cause of the disease is mostly sporadic with much less familial. In familial cases of Alzheimer's disease, the mutations in genes encoding amyloid precursor protein, presenilin 1 and 2, which constitute the catalytic subunits of γ‐secretase, have been identified (Selkoe & Hardy, 2016). In the process of metabolism in normal individual, amyloid precursor protein is digested by α‐secretase following by γ‐secretase, leading to the product which is easily eliminated, while in Alzheimer's disease brain the metabolism by β‐secretase (BACE) and γ‐secretase generates amyloid β‐protein (Aβ1–42), which aggregates and exerts neurotoxicity. Phosphorylated tau protein also causes deposition as neurofibrillary tangles (Reddy & Oliver, 2019).
Neuropolypeptide h3, also known as hippocampal cholinergic neuro‐stimulating peptide, up‐regulates the levels of ChAT whose activity is declined in patients with AD (Ojika, Tsugu, Mitake, Otsuka, & Katada, 1998; Reed, Pierce, Turner, Markesbery, & Butterfield, 2009). Nitration of tyrosine in this peptide decreases its neurotropic activity on cholinergic neurons that may lead to the decline in cognitive function (Reed et al., 2009).
S‐nitrosylation regulates the activity of proteins related to Alzheimer's disease in Alzheimer's disease patients and model animals. The administration of 0.1–100 μM of NO donors deactivated beta‐secretase 1(BACE1) by S‐nitrosylation in rat primary cortical neurons, and the levels of S‐nitrosylated beta‐secretase 1 are decreased in human Alzheimer's disease brains (Kwak et al., 2011). The activity of insulin degrading enzyme, which is a zinc metalloendopeptidase responsible for the metabolism of Aβ1–42 as well as insulin, is suppressed by S‐nitrosylation in Alzheimer's disease brain (Akhtar et al., 2016).
The levels of beta‐secretase 1, presenilin1 and pp38 MAPK are increased while those of disintegrin and metalloproteinase domain‐containing protein 17 (ADAM17) are decreased in the amyloid precursor protein/ presenilin1 transgenic mice (He et al., 2016). The administration of NaHS into the transgenic mice restores the changes in these factors characteristic for Alzheimer's disease, suggesting that H2S inhibits the expression of beta‐secretase 1 and presenilin1 by activating PI3K/Akt pathway in Alzheimer's disease (He et al., 2016).
H2S inhibited exogenous ATP‐induced inflammatory responses through reducing pro‐inflammatory cytokines, ROS, and activation of NF‐κB pathway. H2S also suppressed the production of Aβ1–42, which was induced by exogenous ATP probably due to the augmented production of amyloid precursor protein and the activation of β‐ and γ‐secretase (Cao et al., 2018) (Figure 5b). As a mechanism for H2S reducing inflammation and the production of Aβ1–42, they suggested that H2S suppresses the activities of STAT3 by inhibiting ATP‐induced phosphorylation and decreases the activity of cathepsin S through S‐sulfuration of this enzyme (Cao et al., 2018) (Figure 5b).
8. H2S AND H2Sn ARE BENEFICIAL OR TOXIC
8.1. Schizophrenia
Schizophrenia is a chronic and severe mental disorder that affects a person's thinking, feeling, and behaviours. Symptoms, which typically come on gradually and begin in young adulthood, fall into three categories: positive symptoms (hallucinations, delusions, etc.), negative symptoms (flat affect, reduced feeling etc.), and cognitive symptoms (poor executive function, trouble focusing etc.) (American Psychiatric Association, 2013).
Although the excessive NO production has been shown to be involved in the pathology of this disease (Pitsikas, 2016), the potential beneficial effects have been reported for both NO donors and inhibitors on schizophrenia symptoms induced by amphetamine such as prepulse inhibition disruption and hyperlocomotion (Issy, Dos‐Santos‐Pereira, Pedrazzi, Kubrusly, & Del‐Bel, 2018), suggesting that the deviation of both decrease and increase of NO from the normal levels may be involved in the pathology of this disease.
Both excess and deficiency of H2S and H2Sn have also been proposed to be involved in the pathogenesis of schizophrenia. Plasma H2S levels were significantly lower in patients with schizophrenia relative to healthy control subjects, and a positive association was observed between plasma H2S levels and working memory, visual memory, or executive function in patients, suggesting that decreased H2S is involved in the psychopathology and cognitive deficits of this disease (Xiong et al., 2018) (Figure 5b).
Untreated schizophrenia patients had significantly higher (disulfide/total thiol) and (disulfide/free thiol) ratio in blood and a significantly lower (free thiol/total thiol) ratio compared to those of healthy individuals (Figure 5b). Thiol homeostasis is disturbed by a shift to the disulfide bond formation (oxidized) in patients (Topcuoglu et al., 2017). Similar results were also obtained in schizophrenia patients using medication (Ünal et al., 2018).
Methylglyoxal, a highly reactive dicarbonyl compound, is a major precursor for advanced glycation end products, production of which is associated with various neurological disorders including schizophrenia (Ohnishi et al., 2019; Toyoshima et al., 2019). H2Sn protect differentiated human neuroblastoma SH‐SY5Y cells from methylglyoxal‐induced cytotoxicity, suggesting that H2Sn scavenge methylglyoxal and suppress the accumulation of advanced glycation end products and incidents induced by carbonyl stress (Koike, Ogasawara, Shibuya, Kimura, & Ishii, 2013).
Excess production of H2S and H2Sn has also been proposed to be involved in the pathogenesis of schizophrenia. Yoshikawa and colleagues found that C57BL/6N (B6) strain mice exhibited greater scores of prepulse inhibition, which is the normal suppression of a startle response, than C3H/HeN (C3H) mice did (Watanabe et al., 2007). The impaired prepulse inhibition is regarded as an endophenotype for schizophrenia (Braff, Geyer, & Swerdlow, 2001). By proteomic analysis, the same group found that the expression of MPST is increased in C3H mice compared to B6 (Ide et al., 2019). DNA methylation levels at MPST gene were highly enhanced in C3H mice, and the mean methylation levels of the sites were positively correlated with the expression levels of MPST. MPST levels in schizophrenia were positively correlated with symptom severity scores. In Mpst‐transgenic mice, the expression of genes for energy formation was decreased and mitochondrial energy metabolism was impaired (Ide et al., 2019) (Figure 5a).
Maternal immune activation model, which is induced by the injection of polyriboinosinic‐polyribocytidylic acid to mother, shows perturbed early neural development via inflammation and oxidative insults (Bundo et al., 2014; Giovanoli et al., 2013; Meyer & Feldon, 2012). In this model, the expression of MPST and other inflammatory and oxidative genes was elevated in the brain when pups grow to the adult (Ide et al., 2019). In human brains, catalase gene product was up‐regulated in schizophrenia samples compared to the controls (Ide et al., 2019). Inflammatory/oxidative insults in early brain development induce up‐regulation of H2S/polysulfides production excessively as an antioxidative response that suppresses cytochrome c oxidase, leading to schizophrenia.
9. PERSPECTIVE
Neurotransmitters, cytokines and hormones diffuse to reach their targets to react with their receptors. An advantage of diffusion is that many targets localized in the area within its reach can be activated. After transmitting the signal, they are properly eliminated. For example neurotransmitters that are released from presynaptically and diffuse across the synaptic cleft to the postsynaptic receptors to exert their effects. For cessation of the responses the transmitters are recovered by uptake or degraded by enzymes to clear the synaptic cleft.
NO barely dissolves in water (5.6 mg·100 ml−1 at 20°C), while H2S does well (413 mg·100 ml−1 at 20°C) (Kimura, 2015b). H2S dissociates to H+ and HS− (pK1 = 7.04) and further to S2− (pK2 = 11.96). Because H2S is also lipophilic, it readily passes through plasma membrane. These characteristics of H2S have an advantage to transmit signals across the membrane by diffusion. HS− channels or transporters, which have been identified in both bacteria and mammals (Czyzewski & Wang, 2012; Jennings, 2013), enable H2S to pass through plasma membrane even more efficiently and selectively.
H2Sn, which can pass through the plasma membrane, diffuse to the targets on nearby cells (Greiner et al., 2013; Kimura et al., 2017; Kimura et al., 2013; Nagai et al., 2006; Oosumi et al., 2010). For example, H2Sn activate TRPA1 channels by passing through the plasma membrane to S‐sulfurate the amino terminus located in the cytoplasm (Kimura et al., 2013; Nagai et al., 2006; Oosumi et al., 2010). Although 23 out of 31 cysteine residues are localized to the amino terminus (Wang, Cvetkov, Chance, & Moiseenkova‐Bell, 2012), only Cys422 and Cys634 are responsible for regulating the activity of TRPA1 channels (Hatakeyama et al., 2015; Kimura, 2015a). The remaining 21 cysteine residues were not involved in the activation of channels whether or not they are S‐sulfurated. Signalling by diffusion may be less specific to target proteins than that by enzymatic modification. In this case, however, S‐sulfuration of the two specific cysteine residues by sulfur transferases, if any, may not have any advantage on specificity to activate the channels compared to the diffusion of H2Sn.
S‐sulfuration is compared to phosphorylation, in which kinases incorporate phosphate to residues of serine, threonine and tyrosine (Kimura, 2020). H2S and H2Sn are able to S‐sulfurate cysteine residues of proteins even in cells surrounding the producing cells but do not S‐sulfurate the specific residues. In contrast, MPST transfers sulfur from 3MP to specific cysteine residues, but the reaction is restricted only inside the cells (Kimura et al., 2017; Shibuya et al., 2013; Shibuya et al., 2009). H2S and H2Sn endogenously exist (Kimura et al., 2015; Koike et al., 2017) and both their diffusion‐ and enzyme‐mediated mechanisms play a role in S‐sulfuration under physiological conditions. Bacteria use H2S produced by MPST to protect themselves from antibiotics (Shatalin, Shatalina, Mironov, & Nudler, 2011). Because bacteria have channels specific to HS− (Czyzewski & Wang, 2012), signalling by H2S with other cells must have emerged in the early history of life on earth. Mammalian have developed HS−/Cl− transporter on erythrocytes to rapidly and selectively exchange HS− with the extracellular milieu (Jennings, 2013).
Patients exposed to high concentrations of H2S suffer from cognitive impairment (Reiffenstein, Hulbert, & Roth, 1992) and the levels of neurotransmitters in the brain were affected in animals exposed to H2S (Warenycia, Smith, Blashko, Kombian, & Reiffenstein, 1989). The expression of sulfur quinone oxidoreductase, which is the first step for the metabolism of H2S, is very low in the brain (Linden et al., 2012). These observations suggest the vulnerability of the brain to excessive H2S.
In Down's syndrome, ethylmalonyl encephalopathy and schizophrenia, the high levels of H2S or its producing enzymes exert a toxic effect on cytochrome c oxidase, leading to neuronal dysfunction (Ichinohe et al., 2005; Ide et al., 2019; Linden et al., 2012; Panagaki et al., 2019; Taub et al., 1999; Tiranti et al., 2009; Viscomi et al., 2010). In addition, high concentrations of H2S metabolized by sulfur quinone oxidoreductase must generate the toxic levels of polysulfides on neurons. The inhibitors of H2S producing enzymes, which may also decrease the levels of polysulfides, have suggested to have therapeutic potential in these diseases. The accurate comparisons of the levels of H2S and polysulfides between patients and normal individuals are required to examine the efficiency of these inhibitors for therapeutic uses.
S‐sulfuration makes parkin active in the brains of normal individuals, while S‐nitrosylation inactivates it in those with Parkinson's disease (Vandiver et al., 2013). It is intriguing to know whether or not exogenously applied H2S or the enhancement of H2S producing enzymes is able to convert S‐nitrosylated cysteine to S‐sulfurated one. The difference in the levels of H2S between patients and normal individuals is not well understood in Parkinson's disease. The concentrations of polysulfides, which must be accompanied by the changes of H2S levels, should also be clarified. Controlling the levels of these signalling molecules and the activities of enzymes related to S‐nitrosylation and S‐sulfuration may have a therapeutic benefit for diseases in the CNS.
9.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
CONFLICT OF INTEREST
The author declared no conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported by the KAKENHI (17K08331), a Grant‐in‐Aid for Scientific Research, and the Strategic Research Program for Brain Sciences from the Japan Agency for Medical Research and Development, AMED, under Grant JP20dm0107085 to H.K.
Kimura H. Hydrogen sulfide signalling in the CNS ‐ Comparison with NO. Br J Pharmacol. 2020;177:5031–5045. 10.1111/bph.15246
REFERENCES
- Abe, K. , & Kimura, H. (1996). The possible role of hydrogen sulfide as an endogenous neuromodulator. The Journal of Neuroscience, 16, 1066–1071. 10.1523/JNEUROSCI.16-03-01066.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou‐Hamdan, A. , Ransy, C. , Roger, T. , Guedouari‐Bounihi, H. , Galardon, E. , & Bouillaud, F. (2016). Positive feedback during sulfide oxidation fine‐tunes cellular affinity for oxygen. Biochimica et Biophysica Acta, 1857, 1464–1472. 10.1016/j.bbabio.2016.04.282 [DOI] [PubMed] [Google Scholar]
- Aizenman, E. , Lipton, D. A. , & Loring, R. H. (1989). Selective modulation of NMDA responses by reduction and oxidation. Neuron, 2, 1257–1263. 10.1016/0896-6273(89)90310-3 [DOI] [PubMed] [Google Scholar]
- Akhtar, M. W. , Sanz‐Blasco, S. , Dolatabadi, N. , Parker, J. , Chon, K. , Lee, M. S. , … Lipton, S. A. (2016). Elevated glucose and oligomeric β‐amyloid disrupt synapses via a common pathway of aberrant protein S‐nitrosylation. Nature Communications, 7, 10242–10252Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4729876/pdf/ncomms10242.pdf. 10.1038/ncomms10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , & CGTP Collaborators (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176 Suppl 1(Suppl 1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Arnold, W. P. , Mittal, C. K. , Katsuki, S. , & Murad, F. (1977). Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′‐cyclic monophosphate levels in various tissue preparations. Proceedings of the National Academy of Sciences of the United States of America, 74, 3203–3207. 10.1073/pnas.74.8.3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff, D. L. , Geyer, M. A. , & Swerdlow, N. R. (2001). Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology, 156, 234–258. 10.1007/s002130100810 [DOI] [PubMed] [Google Scholar]
- Bredt, D. S. , Hwang, P. M. , Glatt, C. E. , Lowenstein, C. , Reed, R. R. , & Snyder, S. H. (1991). Cloned and expressed nitric oxide synthase structurally resembles cytochrome P‐450 reductase. Nature, 351, 714–718. [DOI] [PubMed] [Google Scholar]
- Bredt, D. S. , & Snyder, S. H. (1990). Isolation of nitric oxide synthetase, a calmodulin‐requiring enzyme. Proceedings of the National Academy of Sciences of the United States of America, 87, 682–685. 10.1073/pnas.87.2.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundo, M. , Toyoshima, M. , Okada, Y. , Akamatsu, W. , Ueda, J. , Nemoto‐Miyauchi, T. , & Iwamoto, K. (2014). Increased l1 retrotransposition in the neuronal genome in schizophrenia. Neuron, 81, 306–313. [DOI] [PubMed] [Google Scholar]
- Cao, L. , Cao, X. , Zhou, Y. , Nagpure, B. V. , Wu, Z. Y. , Hu, L. F. , … Bian, J. S. (2018). Hydrogen sulfide inhibits ATP‐induced neuroinflammation and Aβ1‐42 synthesis by suppressing the activation of STAT3 and cathepsin S. Brain, Behavior, and Immunity, 73, 603–614. 10.1016/j.bbi.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Cepeda, C. , & Tong, X. P. (2018). Huntington's disease: From basic science to therapeutics. CNS Neuroscience & Therapeutics, 24, 247–249. 10.1111/cns.12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti, S. S. , McCusker, E. A. , & Loy, C. T. (2017). What do we know about late onset Huntington's disease? Journal of Huntington's Disease, 6, 95–103. 10.3233/JHD-170247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Suzuki, H. , & Weston, A. H. (1988). Acetylcholine releases endothelium‐derived hyperpolarizing factor and EDRF from rat blood vessels. British Journal of Pharmacology, 95, 1165–1174. 10.1111/j.1476-5381.1988.tb11752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiku, T. , Padovani, D. , Zhu, W. , Singh, S. , Vitvitsky, V. , & Banerjee, R. (2009). H2S biogenesis by human cystathionine γ‐lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. The Journal of Biological Chemistry, 284, 11601–11612. 10.1074/jbc.M808026200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, D. W. (1988). Glutamate neurotoxicity and diseases of the nervous system. Neuron, 1, 623–634. 10.1016/0896-6273(88)90162-6 [DOI] [PubMed] [Google Scholar]
- Choi, P. , Golts, N. , Snyder, H. , Chong, M. , Petrucelli, L. , Hardy, J. , … Wolozin, B. (2001). Co‐association of parkin and α‐synuclein. Neuroreport, 12, 2839–2843. 10.1097/00001756-200109170-00017 [DOI] [PubMed] [Google Scholar]
- Choi, Y. B. , Tenneti, L. , Le, D. A. , Ortiz, J. , Bai, G. , Chen, H. S. , & Lipton, S. A. (2000). Molecular basis of NMDA receptor‐coupled ion channel modulation by S‐nitrosylation. Nature Neuroscience, 3, 15–21. 10.1038/71090 [DOI] [PubMed] [Google Scholar]
- Chung, K. K. , Thomas, B. , Li, X. , Pletnikova, O. , Troncoso, J. C. , Marsh, L. , … Dawson, T. M. (2004). S‐nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science, 304, 1328–1331. 10.1126/science.1093891 [DOI] [PubMed] [Google Scholar]
- Clarke, H. T. (1932). The action of sulfite upon cystine. The Journal of Biological Chemistry, 97, 235–248. [Google Scholar]
- Contestabile, A. , & Ciani, E. (2004). Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochemistry International, 45, 903–914. 10.1016/j.neuint.2004.03.021 [DOI] [PubMed] [Google Scholar]
- Cortese‐Krott, M. M. , Kuhnle, G. G. C. , Dyson, A. , Fernandez, B. O. , Grman, M. , DuMond, J. F. , … Feelisch, M. (2015). Key bioactive reaction products of the NO/H2S interaction are S/N‐hybrid species, polysulfides, and nitroxyl. Proceedings of the National Academy of Sciences of the United States of America, 112, E4651–E4660. 10.1073/pnas.1509277112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzewski, B. K. , & Wang, D. N. (2012). Identification and characterization of a bacterial hydrosulphide ion channel. Nature, 483, 494–497. 10.1038/nature10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dóka, É. , Ida, T. , Dagnell, M. , Abiko, Y. , Luong, N. C. , Balog, N. , … Nagy, P. (2020). Control of protein function through oxidation and reduction of persulfidated states. Science Advances, 6, eaax8358 10.1126/sciadv.aax8358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt, M. , Dux, M. , Namer, B. , Jiljkovic, J. , Cordasic, N. , Will, C. , … Filipovic, M. R. (2014). H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO–TRPA1–CGRP signaling pathway. Nature Communications, 5, 4381 10.1038/ncomms5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido, Y. , Suzuki, E. , Iwasawa, K. , Namekata, K. , Okazawa, H. , & Kimura, H. (2005). Cystathionine β‐synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. The FASEB Journal, 19, 1854–1856. 10.1096/fj.05-3724fje [DOI] [PubMed] [Google Scholar]
- Fernandez Cardoso, G. M. , Pletsch, J. T. , Parmeggiani, B. , Grings, M. , Glanzel, N. M. , Bobermin, L. D. , … Leipnitz, G. (2017). Bioenergetics dysfunction, mitochondrial permeability transition pore opening and lipid peroxidation induced by hydrogen sulfide as relevant pathomechanisms underlying the neurological dysfunction characteristic of ethylmalonic encephalopathy. Biochimica et Biophysica Acta ‐ Molecular Basis of Disease, 1863, 2192–2201. 10.1016/j.bbadis.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Filipovic, M. R. , Miljkovic, J. L. , Nauser, T. , Royzen, M. , Klos, K. , Shubina, T. , … Ivanović‐Burmazović, I. (2012). Chemical characterization of the smallest S‐nitrosothiol, HSNO; cellular cross‐talk of H2S and S‐nitrosothiols. Journal of the American Chemical Society, 134, 12016–12027. 10.1021/ja3009693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott, R. F. , & Zawadzki, J. V. (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature, 288, 373–376. 10.1038/288373a0 [DOI] [PubMed] [Google Scholar]
- Garthwaite, J. (1991). Glutamate, nitric oxide and cell‐cell signalling in the nervous system. Trends in Neurosciences, 14, 60–67. 10.1016/0166-2236(91)90022-m [DOI] [PubMed] [Google Scholar]
- Garthwaite, J. , Charles, S. L. , & Chess Williams, R. (1988). Endothelium‐derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature, 336, 385–388. 10.1038/336385a0 [DOI] [PubMed] [Google Scholar]
- Giovanoli, S. , Engler, H. , Engler, A. , Richetto, J. , Mareike, V. , Roman, W. , … Urs, M. (2013). Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science, 339, 1095–1099. 10.1126/science.1228261 [DOI] [PubMed] [Google Scholar]
- Goodwin, L. R. , Francom, D. , Dieken, F. P. , Taylor, J. D. , Warenycia, M. W. , Reiffenstein, R. J. , & Dowling, G. (1989). Determination of sulfide in brain tissue by gas dialysis/ion chromatography: Postmortem studies and two case reports. Journal of Analytical Toxicology, 13, 105–109. 10.1093/jat/13.2.105 [DOI] [PubMed] [Google Scholar]
- Goubern, M. , Andriamihaja, M. , Nübel, T. , Blachier, F. , & Bouillaud, F. (2007). Sulfide, the first inorganic substrate for human cells. The FASEB Journal, 21, 1699–1706. 10.1096/fj.06-7407com [DOI] [PubMed] [Google Scholar]
- Greiner, R. , Palinkas, Z. , Basell, K. , Becher, D. , Antelmann, H. , Nagy, P. , & Dick, T. P. (2013). Polysulfides link H2S to protein thiol oxidation. Antioxidants and Redox Signaling, 19, 1749–1765. 10.1089/ars.2012.5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama, Y. , Takahashi, K. , Tominaga, M. , Kimura, H. , & Ohta, T. (2015). Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. Molecular Pain, 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. L. , Yan, N. , Chen, X. S. , Qi, Y. W. , Yan, Y. , & Cai, Z. (2016). Hydrogen sulfide down‐regulates BACE1 and PS1 via activating PI3K/Akt pathway in the brain of APP/PS1 transgenic mouse. Pharmacological Reports, 68, 975–982. 10.1016/j.pharep.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Hildebrandt, T. M. , Meo, I. D. , Zeviani, M. , Viscomi, C. , & Braun, H. P. (2013). Proteome adaptations in Ethe1‐deficient mice indicate a role in lipid catabolism and cytoskeleton organization via post‐translational protein modifications. Bioscience Reports, 33, e00052 10.1042/BSR20130051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, B. C. , Woon, T. C. , Nicholls, P. , Peterson, J. , Greenwood, C. , & Thomson, A. J. (1984). Interactions of sulphide and other ligands with cytochrome c oxidase. An electron‐paramagnetic‐resonance study. Biochemical Journal, 224, 591–600. 10.1042/bj2240591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiike, K. , Tojo, H. , Arai, R. , Nozaki, M. , & Maeda, T. (1994). d‐Amino‐acid oxidase is confined to the lower brain stem and cerebellum in rat brain: Regional differentiation of astrocytes. Brain Research, 652, 297–303. 10.1016/0006-8993(94)90240-2 [DOI] [PubMed] [Google Scholar]
- Hosoki, R. , Matsuki, N. , & Kimura, H. (1997). The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and Biophysical Research Communications, 237, 527–531. 10.1006/bbrc.1997.6878 [DOI] [PubMed] [Google Scholar]
- Hu, L. F. , Lu, M. , Tiong, C. X. , Dawe, G. S. , Hu, G. , & Bian, J. S. (2010). Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell, 9, 135–146. 10.1111/j.1474-9726.2009.00543.x [DOI] [PubMed] [Google Scholar]
- Ichinohe, A. , Kanaumi, T. , Takashima, S. , Enokido, Y. , Nagai, Y. , & Kimura, H. (2005). Cystathionine β‐synthase is enriched in the brains of Down's patients. Biochemical and Biophysical Research Communications, 338, 1547–1550. 10.1016/j.bbrc.2005.10.118 [DOI] [PubMed] [Google Scholar]
- Ide, M. , Ohnishi, T. , Toyoshima, M. , Balan, S. , Maekawa, M. , Shimamoto‐Mitsuyama, C. , … Yoshikawa, T. (2019). Excess hydrogen sulfide and polysulfides production underlies a schizophrenia pathophysiology. EMBO Molecular Medicine, 11, e10695 10.15252/emmm.201910695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro, L. J. , Buga, G. M. , Wood, K. S. , Byrns, R. E. , & Chaudhuri, G. (1987). Endothelium‐derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences of the United States of America, 84, 9265–9269. 10.1073/pnas.84.24.9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami, M. , Hiraki, K. , Umemura, K. , Ogasawara, Y. , Ishii, K. , & Kimura, H. (2009). A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxidants and Redox Signaling, 11, 205–214. 10.1089/ars.2008.2132 [DOI] [PubMed] [Google Scholar]
- Ishii, I. , Akahoshi, N. , Yu, X. N. , Kobayashi, Y. , Namekata, K. , Komaki, G. , & Kimura, H. (2004). Murine cystathionine γ‐lyase: Complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochemical Journal, 381, 113–123. 10.1042/BJ20040243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issy, A. C. , Dos‐Santos‐Pereira, M. , Pedrazzi, J. F. C. , Kubrusly, R. C. C. , & Del‐Bel, E. (2018). The role of striatum and prefrontal cortex in the prevention of amphetamine‐induced schizophrenia‐like effects mediated by nitric oxide compounds. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 86, 353–362. 10.1016/j.pnpbp.2018.03.015 [DOI] [PubMed] [Google Scholar]
- Jaffrey, S. R. , Erdjument‐Bromage, H. , Ferris, C. D. , Tempst, P. , & Snyder, S. H. (2001). Protein S‐nitrosylation: A physiological signal for neuronal nitric oxide. Nature Cell Biology, 3, 193–197. 10.1038/35055104 [DOI] [PubMed] [Google Scholar]
- Jarosz, A. P. , Wei, W. , Gauld, J. W. , Auld, J. , Ozcan, F. , Aslan, M. , & Mutus, B. (2015). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) is inactivated by S‐sulfuration in vitro . Free Radical Biology and Medicine, 89, 512–521. 10.1016/j.freeradbiomed.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Jennings, M. L. (2013). Transport of H2S and HS− across the human red blood cell membrane: Rapid H2S diffusion and AE1‐mediated Cl−/HS− exchange. American Journal of Physiology. Cell Physiology, 305, C941–C950. 10.1152/ajpcell.00178.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jęśko, H. , Lenkiewicz, A. M. , Wilkaniec, A. , & Adamczyk, A. (2019). The interplay between parkin and alpha‐synuclein; possible implications for the pathogenesis of Parkinson's disease. Acta Neurobiologiae Experimentalis (Wars), 79, 276–289. [PubMed] [Google Scholar]
- Kamoun, P. , Belardinelli, M. C. , Chabli, A. , Lallouchi, K. , & Chadefaux‐Vekemans, B. (2003). Endogenous hydrogen sulfide overproduction in Down syndrome. American Journal of Medical Genetics. Part a, 116A, 310–311. 10.1002/ajmg.a.10847 [DOI] [PubMed] [Google Scholar]
- Kimura, H. (2015a). Signaling molecules: Hydrogen sulfide and polysulfide. Antioxidants and Redox Signaling, 22, 362–376. 10.1089/ars.2014.5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, H. (2015b). Hydrogen sulfide and polysulfides as signaling molecules. Proceedings of the Japan Academy, Series B, 91, 131–159. 10.2183/pjab.91.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, H. (2016). Hydrogen polysulfide (H2Sn) signaling along with hydrogen sulfide (H2S) and nitric oxide (NO). Journal of Neural Transmission, 123, 1235–1245. 10.1007/s00702-016-1600-z [DOI] [PubMed] [Google Scholar]
- Kimura, H. (2020). Signalling by hydrogen sulfide and polysulfides via protein S‐sulfuration. British Journal of Pharmacology, 177, 720–733. 10.1111/bph.14579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y. , Dargusch, R. , Schubert, D. , & Kimura, H. (2006). Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxidants and Redox Signaling, 8, 661–670. 10.1089/ars.2006.8.661 [DOI] [PubMed] [Google Scholar]
- Kimura, Y. , Goto, Y.‐I. , & Kimura, H. (2010). Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxidants and Redox Signaling, 12, 1–13. 10.1089/ars.2008.2282 [DOI] [PubMed] [Google Scholar]
- Kimura, Y. , & Kimura, H. (2004). Hydrogen sulfide protects neurons from oxidative stress. The FASEB Journal, 18, 1165–1167. 10.1096/fj.04-1815fje [DOI] [PubMed] [Google Scholar]
- Kimura, Y. , Koike, S. , Shibuya, N. , Lefer, D. , Ogasawara, Y. , & Kimura, H. (2017). 3‐Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine‐ and glutathione‐persulfide (Cys‐SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Scientific Reports, 7, 10459 10.1038/s41598-01711004-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y. , Mikami, Y. , Osumi, K. , Tsugane, M. , Oka, J.‐I. , & Kimura, H. (2013). Polysulfides are possible H2S‐derived signaling molecules in rat brain. The FASEB Journal, 27, 2451–2457. 10.1096/fj.12-226415 [DOI] [PubMed] [Google Scholar]
- Kimura, Y. , Shibuya, N. , & Kimura, H. (2019). Sulfite protects neurons from oxidative stress. British Journal of Pharmacology, 176, 571–582. 10.1111/bph.14373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y. , Toyofuku, Y. , Koike, S. , Shibuya, N. , Nagahara, N. , Lefer, D. , … Kimura, H. (2015). Identification of H2S3 and H2S produced by 3‐mercaptopyruvate sulfurtransferase in the brain. Scientific Reports, 5, 14774 10.1038/srep14774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A. L. , Polhemus, D. , Bhushan, S. , Otsuka, H. , Kondo, K. , Nicholson, C. K. , & Lefer, D. J. (2014). Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase–nitric oxide dependent. Proceedings of the National Academy of Sciences of the United States of America, 111, 3182–3187. 10.1073/pnas.1321871111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike, S. , Kawamura, K. , Kimura, Y. , Shibuya, N. , Kiimura, H. , & Ogasawara, Y. (2017). Analysis of endogenous H2S and H2Sn in mouse brain by high performance liquid chromatography with fluorescence and tandem mass spectrometric detection. Free Radical Biology and Medicine, 113, 355–362. 10.1016/j.freeradbiomed.2017.10.346 [DOI] [PubMed] [Google Scholar]
- Koike, S. , Ogasawara, Y. , Shibuya, N. , Kimura, H. , & Ishii, K. (2013). Polysulfide exerts a protective effect against cytotoxicity caused by t‐buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Letters, 587, 3548–3555. 10.1016/j.febslet.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Kondo, K. , Bhushan, S. , King, A. L. , Prabhu, S. D. , Hamid, T. , Koenig, S. , … Lefer, D. J. (2013). H2S protects against pressure overload‐induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation, 127, 1116–1127. 10.1161/CIRCULATIONAHA.112.000855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. , Dejanovic, B. , Hetsh, F. , Semtner, M. , Fusca, D. , Arjune, S. , … Kloppenburg, P. (2018). S‐sulfocysteine/NMDA receptor‐dependent signaling underlies neurodegeneration in molybdenum cofactor deficiency. The Journal of Clinical Investigation, 127, 4365–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak, Y.‐D. , Wang, R. , Li, J. J. , Zhang, Y.‐W. , Xu, H. , & Liao, F.‐F. (2011). Differential regulation of BACE1 expression by oxidative and nitrosative signals. Molecular Neurodegeneration, 6(1), 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagoutte, E. , Mimoun, S. , Andriamihaja, M. , Chaumontet, C. , Blachier, F. , & Bouillaud, F. (2010). Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochimica et Biophysica Acta, 1797, 1500–1511. 10.1016/j.bbabio.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Lancaster, J. R. Jr. (2017). How are nitrosothiols formed de novo in vivo? Archives of Biochemistry and Biophysics, 617, 137–144. 10.1016/j.abb.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Lewerenz, J. , Ates, G. , Methner, A. , Conrad, M. , & Maher, P. (2018). Oxytosis/ferroptosis (re‐)emerging roles for oxidative stress‐dependent non‐apoptotic cell death in diseases of the central nervous system. Frontiers in Neuroscience, 12, 214 10.3389/fnins.2018.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. L. , Wu, P. F. , C, J. G. , Wang, S. , Han, Q. Q. , Li, D. , … Wang, F. (2017). Activity‐dependent sulfhydration signal controls N‐methyl‐d‐aspartate subtype glutamate receptor‐dependent synaptic plasticity via increasing d‐serine availability. Antioxidants & Redox Signaling, 27, 398–414. 10.1089/ars.2016.6936 [DOI] [PubMed] [Google Scholar]
- Lin, C. T. , & Chen, L. H. (1983). Production and characterization of an antibody to cytosolic aspartate aminotransferase and immunolocalization of the enzyme in rat organs. Laboratory Investigation, 48, 718–725. [PubMed] [Google Scholar]
- Linden, D. R. , Furne, J. , Stoltz, G. J. , Abdel‐Rehim, M. S. , Levitt, M. D. , & Szurszewski, J. H. (2012). Sulphide quinone reductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. British Journal of Pharmacology, 165, 2178–2190. 10.1111/j.1476-5381.2011.01681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, C. C. , Guidotti, A. , & Costa, E. (1974). The regulation of cyclic guanosine monophosphate in rat cerebellum: Possible involvement of putative amino acid neurotransmitters. Brain Research, 79, 510–514. 10.1016/0006-8993(74)90449-1 [DOI] [PubMed] [Google Scholar]
- Marechal, D. , Brault, V. , Leon, A. , Martin, D. , Lopes Pereira, P. , Loaëc, N. , … Herault, Y. (2019). Cbs overdosage is necessary and sufficient to induce cognitive phenotypes in mouse models of Down syndrome and interacts genetically with Dyrk1a . Human Molecular Genetics, 28, 1561–1577. 10.1093/hmg/ddy447 [DOI] [PubMed] [Google Scholar]
- Matsui, T. , Sugiyama, R. , Sakanashi, K. , Tamura, Y. , Iida, M. , Nambu, Y. , … Ikeda‐Saito, M. (2018). Hydrogen sulfide bypasses the rate‐limiting oxygen activation of heme oxygenase. The Journal of Biological Chemistry, 293, 16931–16939. 10.1074/jbc.RA118.004641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino, G. , Bernassola, F. , Knight, R. A. , Corasaniti, M. T. , Nistico, G. , & Finazzi‐Agro, A. (1997). S‐nitrosylation regulates apoptosis. Nature, 388, 432–433. 10.1038/41237 [DOI] [PubMed] [Google Scholar]
- Meyer, U. , & Feldon, J. (2012). To poly(I:C) or not to poly(I:C): Advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology, 62, 1308–1321. 10.1016/j.neuropharm.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Mikami, Y. , Shibuya, N. , Kimura, Y. , Ogasawara, Y. , & Kimura, H. (2011). Thioredoxin and dihydrolipoic acid are endogenous reductants required for 3‐mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. The Biochemical Journal, 439, 479–485. 10.1042/BJ20110841 [DOI] [PubMed] [Google Scholar]
- Minamishima, S. , Bougaki, M. , Sips, P. Y. , Yu, J. D. , Minamishima, Y. A. , Elrod, J. W. , … Ichinose, F. (2009). Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3‐dependent mechanism in mice. Circulation, 120, 888–896. 10.1161/CIRCULATIONAHA.108.833491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishanina, T. V. , Libiad, M. , & Banerjee, R. (2015). Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nature Chemical Biology, 11, 457–464. 10.1038/nchembio.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, R. , Koike, S. , Takano, Y. , Shibuya, N. , Kimura, Y. , Hanaoka, K. , … Kimura, H. (2017). Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Scientific Reports, 7, 45995 10.1038/srep45995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa, A. , & Habara, Y. (2016). Cross talk between polysulfide and nitric oxide in rat peritoneal mast cells. American Journal of Physiology. Cell Physiology, 310, C894–C902. 10.1152/ajpcell.00028.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, T. H. , Miyamoto, M. , Sastre, A. , Schnaar, R. L. , & Coyle, J. T. (1989). Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron, 2, 1547–1558. 10.1016/0896-6273(89)90043-3 [DOI] [PubMed] [Google Scholar]
- Mustafa, A. K. , Gadalla, M. M. , Sen, N. , Kim, S. , Mu, W. , Gazi, S. K. , … Snyder, S. (2009). H2S signals through protein S‐sulfhydration. Science Signaling, 2, ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa, A. K. , Sikka, G. , Gazi, S. K. , Steppan, J. , Jung, S. M. , Bhunia, A. K. , … Snyder, S. H. (2011). Hydrogen sulfide as endothelium‐derived hyperpolarizing factor sulfhydrates potassium channels. Circulation Research, 109, 1259–1268. 10.1161/CIRCRESAHA.111.240242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara, N. (2018). Multiple role of 3‐mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOx production. British Journal of Pharmacology, 175, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara, N. , Ito, T. , Kitamura, H. , & Nishino, T. (1998). Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: Confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochemistry and Cell Biology, 110, 243–250. 10.1007/s004180050286 [DOI] [PubMed] [Google Scholar]
- Nagahara, N. , Koike, S. , Nirasawa, T. , Kimura, H. , & Ogasawara, Y. (2018). Alternative pathway of H2S and polysulfides production from sulfurated catalytic‐cysteine of reaction intermediates of 3‐mercaptopyruvate sulfurtransferase. Biochemical and Biophysical Research Communications, 496, 648–653. 10.1016/j.bbrc.2018.01.056 [DOI] [PubMed] [Google Scholar]
- Nagahara, N. , Nirasawa, T. , Yoshii, T. , & Niimura, Y. (2012). Is novel signal transducer sulfur oxide involved in the redox cycle of persulfide at the catalytic site cysteine in a stable reaction intermediate of mercaptopyruvate sulfurtransferase? Antioxidants & Redox Signaling, 16, 747–753. 10.1089/ars.2011.4468 [DOI] [PubMed] [Google Scholar]
- Nagahara, N. , Yoshii, T. , Abe, Y. , & Matsumura, T. (2007). Thioredoxin dependent enzymatic activation of mercaptopyruvate sulfurtransferase. An intersubunit disulfide bond serves as a redox switch for activation. Journal of Biological Chemistry, 282, 1561–1569. 10.1074/jbc.M605931200 [DOI] [PubMed] [Google Scholar]
- Nagai, Y. , Tsugane, M. , Oka, J. , & Kimura, H. (2004). Hydrogen sulfide induces calcium waves in astrocytes. The FASEB Journal, 18, 557–559. 10.1096/fj.03-1052fje [DOI] [PubMed] [Google Scholar]
- Nagai, Y. , Tsugane, M. , Oka, J.‐I. , & Kimura, H. (2006). Polysulfides induce calcium waves in rat hippocampal astrocytes. Journal of Pharmacological Sciences, 100, 200. [Google Scholar]
- Nakamura, T. , & Lipton, S. A. (2013). Emerging role of protein‐protein transnitrosylation in cell signaling pathways. Antioxidants & Redox Signaling, 18, 239–249. 10.1089/ars.2012.4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell, T. J. , Hawkins, R. D. , Kandel, E. R. , & Arancio, O. (1991). Tests of the roles of two diffusible substances in long‐term potentiation: Evidence for nitric oxide as a possible early retrograde messenger. Proceedings of the National Academy of Sciences USA, 88, 11285–11289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara, Y. , Ishii, K. , Togawa, T. , & Tanabe, S. (1993). Determination of bound sulfur in serum by gas dialysis/high‐performance liquid chromatography. Analytical Biochemistry, 215, 73–81. 10.1006/abio.1993.1556 [DOI] [PubMed] [Google Scholar]
- Ogasawara, Y. , Isoda, S. , & Tanabe, S. (1994). Tissue and subcellular distribution of bound and acid‐labile sulfur, and the enzymic capacity for sulfide production in the rat. Biological and Pharmaceutical Bulletin, 17, 1535–1542. 10.1248/bpb.17.1535 [DOI] [PubMed] [Google Scholar]
- Ohnishi, T. , Balan, S. , Toyoshima, M. , Maekawa, M. , Ohba, H. , Watanabe, A. , … Yoshikawa, T. (2019). Investigation of betaine as a novel psychotherapeutic for schizophrenia. eBioMedicine, 45, 432–446. 10.1016/j.ebiom.2019.05.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojika, K. , Tsugu, Y. , Mitake, S. , Otsuka, Y. , & Katada, E. (1998). NMDA receptor activation enhances the release of a cholinergic differentiation peptide (HCNP) from hippocampal neurons in vitro. Brain Research. Developmental Brain Research, 106, 173–180. 10.1016/s0165-3806(98)00014-5 [DOI] [PubMed] [Google Scholar]
- Olson, K. R. , Gao, Y. , Arif, F. , Arora, K. , Patel, S. , DeLeon, E. R. , … Straub, K. D. (2018). Metabolism of hydrogen sulfide (H2S) and production of reactive sulfur species (RSS) by superoxide dismutase. Redox Biology, 15, 74–85. 10.1016/j.redox.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, K. R. , Gao, Y. , DeLeon, E. R. , Arif, M. , Arif, F. , Arora, N. , & Straub, K. D. (2017). Catalase as a sulfide–sulfur oxido‐reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biology, 12, 325–339. 10.1016/j.redox.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosumi, K. , Tsugane, M. , Ishigami, M. , Nagai, Y. , Iwai, T. , Oka, J.‐I. , & Kimura, H. (2010). Polysulfide activates TRP channels and increases intracellular Ca2+ in astrocytes. Neuroscience Research, 685, e109–e222. [Google Scholar]
- Palmer, R. M. , Ferrige, A. G. , & Moncada, S. (1987). Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature, 327, 524–526. [DOI] [PubMed] [Google Scholar]
- Panagaki, T. , Randi, E. B. , Augsburger, F. , & Szabo, C. (2019). Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in Down syndrome. Proceedings of the National Academy of Sciences of the United States of America, 116, 18769–18771. 10.1073/pnas.1911895116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, B. D. , Sbodio, J. I. , Xu, R. , Vandiver, M. S. , Cha, J. Y. , Snowman, A. M. , & Snyder, S. H. (2014). Cystathionine γ‐lyase deficiency mediates neurodegeneration in Huntington's disease. Nature, 509, 96–100. 10.1038/nature13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsikas, N. (2016). The role of nitric oxide synthase inhibitors in schizophrenia. Current Medicinal Chemistry, 23, 2692–2705. 10.2174/0929867323666160812151054 [DOI] [PubMed] [Google Scholar]
- Reddy, P. H. , & Oliver, D. M. (2019). Amyloid beta and phosphorylated tau‐induced defective autophagy and mitophagy in Alzheimer's disease. Cell, 8, 488 10.3390/cells8050488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, T. T. , Pierce, W. M. Jr. , Turner, D. M. , Markesbery, W. R. , & Butterfield, D. A. (2009). Proteomic identification of nitrated brain proteins in early Alzheimer's disease inferior parietal lobule. Version 2. Journal of Cellular and Molecular Medicine, 13, 2019–2029. 10.1111/j.1582-4934.2008.00478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiffenstein, R. J. , Hulbert, W. C. , & Roth, S. H. (1992). Toxicology of hydrogen sulfide. Annual Review of Pharmacology and Toxicology, 32, 109–134. 10.1146/annurev.pa.32.040192.000545 [DOI] [PubMed] [Google Scholar]
- Ruetz, M. , Kumutima, J. , Lewis, B. E. , Filipovic, M. R. , Lehnert, N. , Stemmler, T. L. , & Banerjee, R. (2017). A distal ligand mutes the interaction of hydrogen sulfide with human neuroglobin. The Journal of Biological Chemistry, 292, 6512–6528. 10.1074/jbc.M116.770370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage, J. C. , & Gould, D. H. (1990). Determination of sulfide in brain tissue and rumen fluid by ion‐interaction reversed‐phase high‐performance liquid chromatography. Journal of Chromatography, 526, 540–545. 10.1016/S0378-4347(00)82537-2 [DOI] [PubMed] [Google Scholar]
- Sbodio, J. I. , Snyder, S. H. , & Paul, B. D. (2016). Transcriptional control of amino acid homeostasis is disrupted in Huntington's disease. Proceedings of the National Academy of Sciences of the United States of America, 113, 8843–8848. 10.1073/pnas.1608264113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbodio, J. I. , Snyder, S. H. , & Paul, B. D. (2018). Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington's disease. Proceedings of the National Academy of Sciences of the United States of America, 115, 780–785. 10.1073/pnas.1717877115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy, D. G. (1996). HS−:O2 oxidoreductase activity of Cu, Zn superoxide dismutase. Archives of Biochemistry and Biophysics, 334, 50–58. 10.1006/abbi.1996.0428 [DOI] [PubMed] [Google Scholar]
- Searcy, D. G. , Whitehead, J. P. , & Maroney, M. J. (1995). Interaction of Cu, Zn superoxide dismutase with hydrogen sulfide. Archives of Biochemistry and Biophysics, 318, 251–263. 10.1006/abbi.1995.1228 [DOI] [PubMed] [Google Scholar]
- Selkoe, D. J. , & Hardy, J. (2016). The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Molecular Medicine, 8, 595–608. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth, D. , Hess, D. T. , Hausladen, A. , Wang, L. , Wang, Y. J. , & Stamler, J. S. (2018). A multiplex enzymatic machinery for cellular protein S‐nitrosylation. Molecular Cell, 69, 451–464.e6. 10.1016/j.molcel.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatalin, K. , Shatalina, E. , Mironov, A. , & Nudler, E. (2011). H2S: A universal defense against antibiotics in bacteria. Science, 334, 986–990. [DOI] [PubMed] [Google Scholar]
- Shibuya, N. , Koike, S. , Tanaka, M. , Ishigami‐Yuasa, M. , Kimura, Y. , Ogasawara, Y. , … Kimura, H. (2013). A novel pathway for the production of hydrogen sulfide from d‐cysteine in mammalian cells. Nature Communications, 4, 1366 10.1038/ncomms2371 [DOI] [PubMed] [Google Scholar]
- Shibuya, N. , Tanaka, M. , Yoshida, M. , Ogasawara, Y. , Togawa, T. , Ishii, K. , & Kimura, H. (2009). 3‐Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants and Redox Signaling, 11, 703–714. 10.1089/ars.2008.2253 [DOI] [PubMed] [Google Scholar]
- Shigetomi, E. , Jackson‐Weaver, O. , Huckstepp, R. T. , O'Dell, T. J. , & Khakh, B. S. (2013). TRPA1 channels are regulators of astrocyte basal calcium levels and long‐term potentiation via constitutive d‐serine release. The Journal of Neuroscience, 33, 10143–10153. 10.1523/JNEUROSCI.5779-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk, M. H. , & Beck, P. W. (1982). Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. The Biochemical Journal, 206, 267–277. 10.1042/bj2060267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng, T. , Axelsson, H. E. , Hedlund, P. , Andersson, D. A. , Jordt, S. E. , Bevan, S. , … Zygmunt, P. M. (2008). Distribution and function of the hydrogen sulfide‐sensitive TRPA1 ion channel in rat urinary bladder. European Urology, 53, 391–399. 10.1016/j.eururo.2007.10.024 [DOI] [PubMed] [Google Scholar]
- Stubbert, D. , Prysyazhna, O. , Rudyk, O. , Scotcher, J. , Burgoyne, J. R. , & Eaton, P. (2014). Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension, 64, 1344–1351. 10.1161/HYPERTENSIONAHA.114.04281 [DOI] [PubMed] [Google Scholar]
- Szabo, C. , Ransy, C. , Módis, K. , Andriamihaja, M. , Murghes, B. , Coletta, C. , … Bouillaud, F. (2014). Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. British Journal of Pharmacology, 171, 2099–2122. 10.1111/bph.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S. , Schubert, D. , & Maher, P. (2001). Oxytosis: A novel form of programmed cell death. Current Topics in Medicinal Chemistry, 1, 497–506. [DOI] [PubMed] [Google Scholar]
- Taqatqeh, F. , Mergia, E. , Neitz, A. , Eysel, U. T. , Koesling, D. , & Mittmann, T. (2009). More than a retrograde messenger: Nitric oxide needs two cGMP pathways to induce hippocampal long‐term potentiation. The Journal of Neuroscience, 29, 9344–9350. 10.1523/JNEUROSCI.1902-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub, J. W. , Huang, X. , Matherly, L. H. , Stout, M. L. , Buck, S. A. , Massey, G. V. , … Ravindranath, Y. (1999). Expression of chromosome 21‐localized genes in acute myeloid leukemia: Differences between Down syndrome and non‐Down syndrome blast cells and relationship to in vitro sensitivity to cytosine arabinoside and daunorubicin. Blood, 94, 1393–1400. [PubMed] [Google Scholar]
- Tiranti, V. , Viscomi, C. , Hildebrandt, T. , Di Meo, I. , Mineri, R. , Tiveron, C. , … Zeviani, M. (2009). Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nature Medicine, 15, 200–205. 10.1038/nm.1907 [DOI] [PubMed] [Google Scholar]
- Toohey, J. I. (2012). The conversion of H2S to sulfane sulfur. Nature Reviews. Molecular Cell Biology, 13, 803author reply p 803. 10.1038/nrm3391-c2 [DOI] [PubMed] [Google Scholar]
- Topcuoglu, C. , Bakirhan, A. , Yilmaz, F. M. , Neselioglu, S. , Erel, O. , & Sahiner, S. Y. (2017). Thiol/disulfide homeostasis in untreated schizophrenia patients. Psychiatry Research, 251, 212–216. 10.1016/j.psychres.2017.02.016 [DOI] [PubMed] [Google Scholar]
- Toyoshima, M. , Jiang, X. , Ogawa, T. , Ohnishi, T. , Yoshihara, S. , Balan, S. , … Hirokawa, N. (2019). Enhanced carbonyl stress induces irreversible multimerization of CRMP2 in schizophrenia pathogenesis. Life Science Alliance, 2, e201900478 10.26508/lsa.201900478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura, K. , & Kimura, H. (2007). Hydrogen sulfide enhances reducing activity in neurons: Neurotrophic role of H2S in the brain? Antioxidants & Redox Signaling, 9, 2035–2041. 10.1089/ars.2007.1802 [DOI] [PubMed] [Google Scholar]
- Ünal, K. , Erzin, G. , Yüksel, R. N. , Alisik, M. , & Erel, Ö. (2018). Thiol/disulphide homeostasis in schizophrenia patients with positive symptoms. Journal of Psychiatry, 72, 281–284. 10.1080/08039488.2018.1441906 [DOI] [PubMed] [Google Scholar]
- Vandini, E. , Ottani, A. , Zaffe, D. , Calevro, A. , Canalini, F. , Cavallini, G. M. , … Giuliani, D. (2019). Mechanisms of hydrogen sulfide against the progression of severe Alzheimer's disease in transgenic mice at different ages. Pharmacology, 103, 50–60. 10.1159/000494113 [DOI] [PubMed] [Google Scholar]
- Vandiver, M. S. , Paul, B. D. , Xu, R. , Karuppagounder, S. , Rao, F. , Snowman, A. M. , … Snyder, S. H. (2013). Sulfhydration mediates neuroprotective actions of parkin. Nature Communications, 4, 1626 10.1038/ncomms2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscomi, C. , Burlina, A. B. , Dweikat, I. , Savoiardo, M. , Lamperti, C. , Hildebrandt, T. , … Zeviani, M. (2010). Combined treatment with oral metronidazole and N‐acetylcysteine is effective in ethylmalonic encephalopathy. Nature Medicine, 16, 869–871. 10.1038/nm.2188 [DOI] [PubMed] [Google Scholar]
- Vitvitsky, V. , Yadav, P. K. , Kurthen, A. , & Banerjee, R. (2015). Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. Journal of Biological Chemistry, 290, 8310–8320. 10.1074/jbc.M115.639831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Cvetkov, T. L. , Chance, M. R. , & Moiseenkova‐Bell, V. Y. (2012). Identification of in vivo disulfide conformation of TRPA1 ion channel. The Journal of Biological Chemistry, 287, 6169–6176. 10.1074/jbc.M111.329748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenycia, M. W. , Goodwin, L. R. , Benishin, C. G. , Reiffenstein, R. J. , Francom, D. M. , Taylor, J. D. , & Dieken, F. P. (1989). Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochemical Pharmacology, 38, 973–981. 10.1016/0006-2952(89)90288-8 [DOI] [PubMed] [Google Scholar]
- Warenycia, M. W. , Goodwin, L. R. , Francom, D. M. , Dieken, F. P. , Kombian, S. B. , & Reiffenstein, R. J. (1990). Dithiothreitol liberates non‐acid labile sulfide from brain tissue of H2S‐poisoned animals. Archives of Toxicology, 64, 650–655. 10.1007/BF01974693 [DOI] [PubMed] [Google Scholar]
- Warenycia, M. W. , Smith, K. A. , Blashko, C. S. , Kombian, S. B. , & Reiffenstein, R. J. (1989). Monoamine oxidase inhibition as a sequel of hydrogen sulfide intoxication: Increases in brain catecholamine and 5‐hydroxytryptamine levels. Archives of Toxicology, 63, 131–136. 10.1007/BF00316435 [DOI] [PubMed] [Google Scholar]
- Watanabe, A. , Toyota, T. , Owada, Y. , Hayashi, T. , Iwayama, Y. , Matsumata, M. , … Yoshikawa, T. (2007). Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. Version 2. PLoS Biology, 5, e297 10.1371/journal.pbio.0050297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman, M. , Li, L. , Kostetski, I. , Chu, S. H. , Siau, J. L. , Bhatia, M. , & Moore, P. K. (2006). Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochemical and Biophysical Research Communications, 343, 303–310. 10.1016/j.bbrc.2006.02.154 [DOI] [PubMed] [Google Scholar]
- Wright, D. J. , Gray, L. J. , Finkelstein, D. I. , Crouch, P. J. , Pow, D. , Pang, T. Y. , … Hannan, A. J. (2016). N‐acetylcysteine modulates glutamatergic dysfunction and depressive behavior in Huntington's disease. Human Molecular Genetics, 25, 2923–2933. 10.1093/hmg/ddw144 [DOI] [PubMed] [Google Scholar]
- Xie, L. , Hu, L. F. , Teo, X. Q. , Tiong, C. X. , Tazzari, V. , Sparatore, A. , … Bian, J. S. (2013). Therapeutic effect of hydrogen sulfide‐releasing l‐Dopa derivative ACS84 on 6‐OHDA‐induced Parkinson's disease rat model. PLoS ONE, 8, e60200 10.1371/journal.pone.0060200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, J. W. , Wei, B. , Li, Y. K. , Zhan, J. Q. , Jiang, S. Z. , Chen, H. B. , … Yang, Y. J. (2018). Decreased plasma levels of gasotransmitter hydrogen sulfide in patients with schizophrenia: Correlation with psychopathology and cognition. Psychopharmacology, 235, 2267–2274. 10.1007/s00213-018-4923-7 [DOI] [PubMed] [Google Scholar]
- Yao, D. , Gu, Z. , Nakamura, T. , Shi, Z. Q. , Ma, Y. , Gaston, B. , … Lipton, S. A. (2004). Nitrosative stress linked to sporadic Parkinson's disease: S‐nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proceedings of the National Academy of Sciences of the United States of America, 101, 10810–10814. 10.1073/pnas.0404161101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, W. L. , Yin, W. G. , Huang, B. S. , & Wu, L. X. (2017). Neuroprotective effects of lentivirus‐mediated cystathionine‐beta‐synthase overexpression against 6‐OHDA‐induced Parkinson's disease rats. Neuroscience Letters, 657, 45–52. 10.1016/j.neulet.2017.07.019 [DOI] [PubMed] [Google Scholar]
- Yong, R. , & Searcy, D. G. (2001). Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology, 129, 129–137. 10.1016/s1096-4959(01)00309-8 [DOI] [PubMed] [Google Scholar]
- Zhao, W. , Zhang, J. , Lu, Y. , & Wang, R. (2001). The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. The EMBO Journal, 20, 6008–6016. 10.1093/emboj/20.21.6008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, M. , Small, S. A. , Kandel, E. R. , & Hawkins, R. D. (1993). Nitric oxide and carbon monoxide produce activity‐dependent long‐term synaptic enhancement in hippocampus. Science, 260, 1946–1950. 10.1126/science.8100368 [DOI] [PubMed] [Google Scholar]
- Zivanovic, J. , Kouroussis, E. , Kohl, J. B. , Adhikari, B. , Bursac, B. , Schott‐Roux, S. , … Filipovic, M. R. (2019). Selective persulfide detection reveals evolutionarily conserved antiaging effects of S‐sulfhydration. Cell Metabolism, 30, 1152–1170.e13. 10.1016/j.cmet.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]


