Scheme 6.
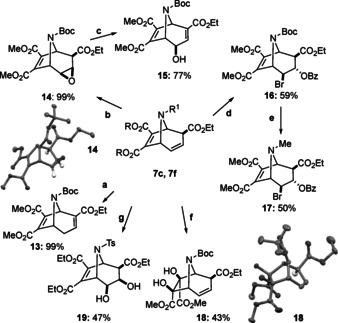
Derivatization reactions of the 8‐azabicyclo[3.2.1]octane framework 7 c and 7 f. Conditions: a) TEA (1.3 equiv), CH2Cl2, 2 h, 99 %; b) mCPBA (4.0 equiv), CH2Cl2, 25 °C, 3 days, 99 %; c) flash chromatography, 1 % TEA, 77 %; d) (i) NBS (2.0 equiv), acetone/H2O (3:1 v/v), 0 to 25 °C, 21 h; (ii) BzCl (1.5 equiv), DMAP (0.5 equiv), TEA (5.0 equiv), CH2Cl2, 25 °C, 8 h, 59 %; e) (i) TFA (33 equiv), CH2Cl2, 25 °C, 1.5 h, (ii) 37 % aq CH2O (6.0 equiv), NaBH3CN (3.0 equiv), MeCN, 25 °C, 1 h, 50 %; f) K2OsO4⋅2 H2O (0.05 equiv), NMO (2.0 equiv), H2O, acetone, 0 °C, 12 h, 43 %; g) RuCl3⋅3 H2O (6 mol %), NaIO4 (1.6 equiv), MeCN, H2O, 0 to 25 °C, 2 days, 47 %.
