Figure 6. The TRPV1 phospho‐acceptor residue S502 mediates the inhibitory effects of 25OHD on PKC‐induced potentiation of TRPV1 activity.
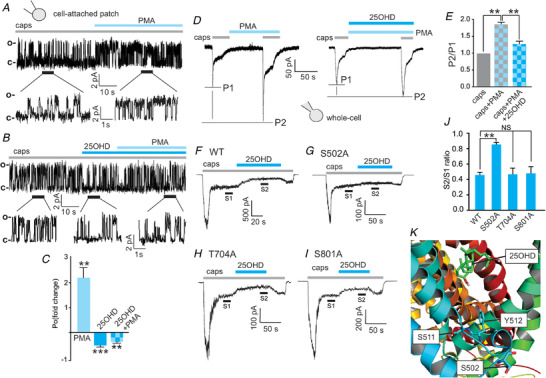
A and B, representative cell‐attached patch single‐channel recordings showing that the PKC activator phorbol 12‐myristate 13‐acetate (PMA, 1 µm) potentiates the increases in open probability of WT TRPV1 single channel currents induced by capsaicin (1 µm) (A) and that co‐application of 25OHD (100 nM) with PMA prevents this potentiation (B). C, grouped data from A and B showing fold changes in open probability (P o) (caps+PMA = 2.2 ± 0.5, caps+25OHD = 0.42 ± 0.1, caps+25OHD+PMA = 0.24 ± 0.2, n = 6 patches per group). D and E, representative whole‐cell current recordings, using a paired pulse protocol, illustrating that PMA potentiates the capsaicin‐elicited current and that 25OHD significantly reduces the PMA potentiating effect. E, grouped data (normalized to capsaicin alone) of the P2/P1 ratio for caps/PMA and caps/PMA/25OHD: 1.8 ± 0.08 and 1.3 ± 0.05, respectively (n = 5–6 cells per group). F–I, representative whole‐cell current traces from WT, S502A, T704A and S800A mutants illustrating that the inhibitory effects of 25OHD on PMA potentiation of sustained TRPV1 currents are significantly reduced only in the S502A mutant. J, normalized grouped data from F–I. S2/S1 ratios for WT, S502A, T704 and S801A are 0.4 ± 0.04, 0.8 ± 0.05, 0.5 ± 0.09 and 0.5 ± 0.08, respectively (n = 5–7 cells per group, mean ± SD). K, human TRPV1 homology 3D model illustrating the predicted locations of S502 in relation to Y511 and S512 and the putative 25OHD binding site. Statistical analysis was performed with a paired Student's t test: * * P < 0.01, * * * P < 0.005 respectively; NS, no significant difference. [Color figure can be viewed at wileyonlinelibrary.com]
