Figure 2.
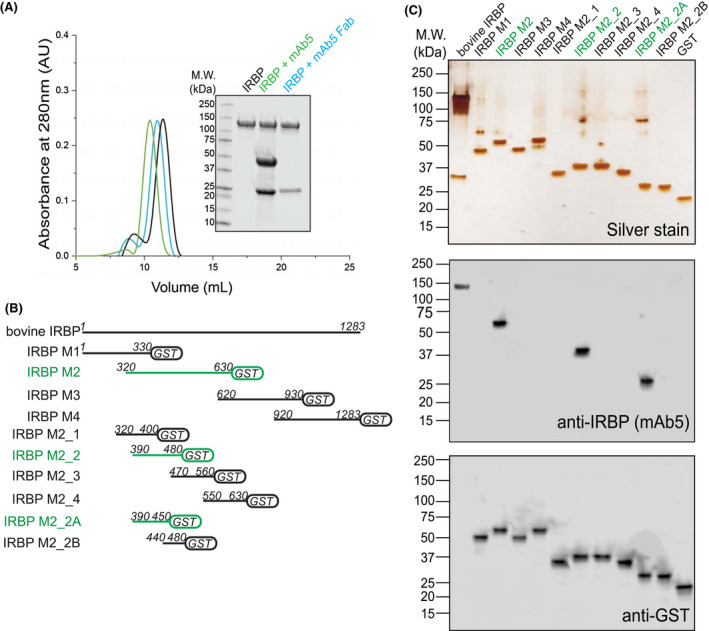
IRBP mAb5 binding characteristics and determination of epitope. A, Both full‐length mAb5 and mAb5 fragment antigen‐binding domain (mAb5 Fab) bind purified bovine IRBP. After incubating IRBP in the presence of either mAb5 or mAb5 Fab in a 1:1 molar ratio, samples were subjected to Superdex‐200 size‐exclusion chromatography. Twenty microgram of eluate peaks was subjected to SDS‐PAGE, and gels were stained with Coomassie blue (inset) showing stable association of both mAb5 and mAb5 Fab. Chromatogram corresponds to IRBP, IRBP + mAb5, and IRBP + mAb5 Fab in black, green, and blue, respectively. B, mAb5 recognizes IRBP M2_2A (residues 390‐450). IRBP Modules and smaller constructs were stably expressed as fusion proteins with glutathione S‐transferase (GST). Primary sequence lengths are represented for each construct, IRBP M1, M2, M3, M4, M2_1, M2_2, M2_3, M2_4, M2_2A, and M2_2B (residue numbers listed in methods and materials). Constructs that bound mAb5 were detected on an immunoblot and highlighted in green. C, Expressed constructs (500 ng) were subjected to SDS‐PAGE and stained by silver stain or transferred for immunoblot purposes. Transferred membranes were probed using mAb5 or an antibody targeting GST (Genscript, Piscataway, NJ, USA) as a primary probe. Purified bovine IRBP and GST were used as positive controls for the antibodies
