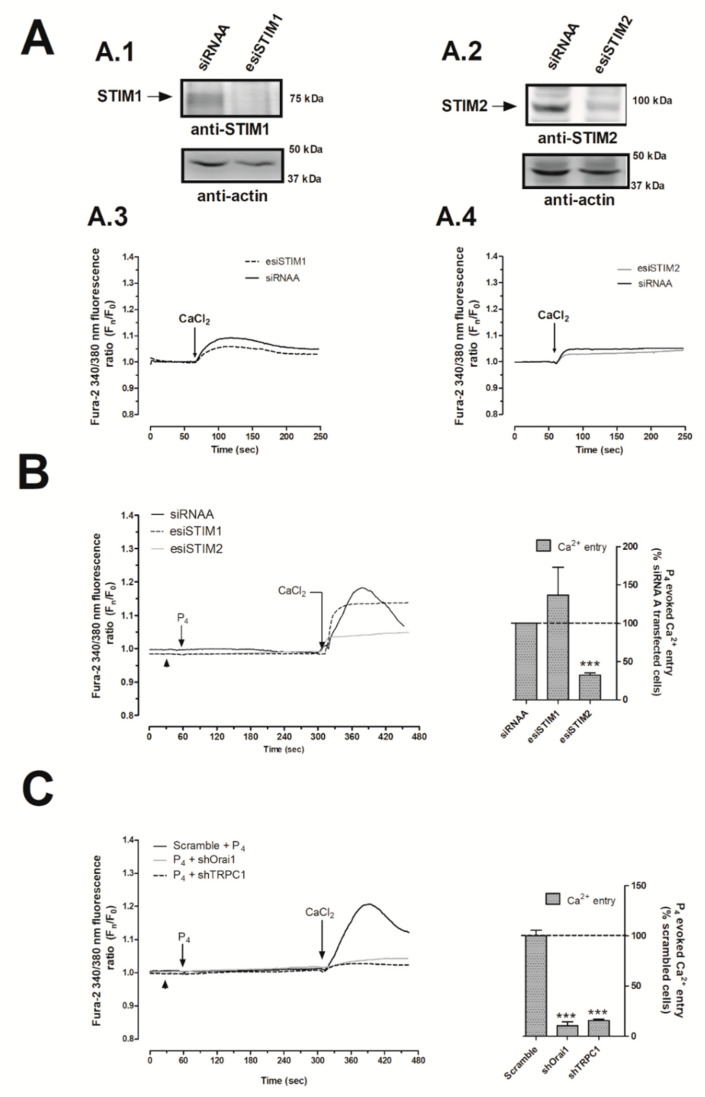
Figure 4.
Progesterone (P4) activated the stromal interaction molecule 2 (STIM2) that drives calcium (Ca2+) entry through Calcium release-activated calcium channel protein 1 (Orai1) and transient receptor potential channel 1 (TRPC1). MDA-MB-231 cells were transfected with 2 μg/mL of either endoribonuclease-generated siRNA against STIM1 (esiSTIM1) (A.1,A.3 (dotted trace) and B) or esiSTIM2 (A.2,A.4 (grey trace) and B) or with a non-specific siRNA (A.1–A.4 (black trace) and B) for 48 h. Alternatively, we used 2 μg/mL of small hairpin RNA against Orai1 (shOrai1) (C, grey solid line) or shTRPC1 (c; black dotted line) or empty vector (C, scrambled cells, black trace) for 48 h. Upon confirmation of the efficiency of the protein silencing protocols by Western blotting using the specific antibodies as indicated (A.1,A.2), Ca2+ experiments were performed according to the procedures described in Materials and Methods. Briefly, fura-2-loaded MDA-MB-231 cells were maintained in a Ca2+-rich medium (containing 1 mM of CaCl2, A.3,A.4) and were kept untreated; while other cells were maintained in a Ca2+-free HBS medium (75 μM of ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) was added, arrowheads (B,C)) and were stimulated with 1 μM of P4 for 4 min (B,C). P4-evoked Ca2+ entry was visualized by adding CaCl2 (1 mM) to the extracellular medium, which was monitored for an additional 2 min. Graphs are representative of 6–8 independent transfections, and the histograms represent the average of the percentage ± Standar error of the Mean (S.E.M.), resulting in the analysis of the areas under the curves corresponding to the Ca2+ entry evoked by P4 in around 60–80 cells from 6–8 independent transfections. ***: represents p < 0.001 with respect to the Ca2+ entry values found in scrambled MDA-MB-231 cells.