Figure 4.
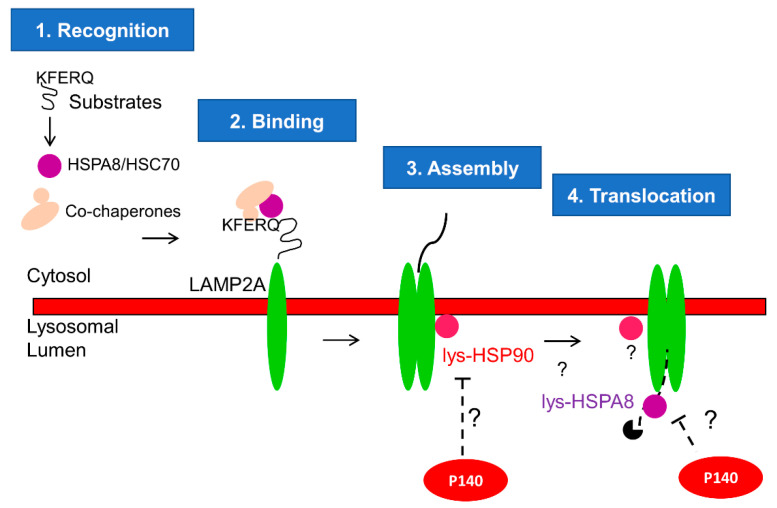
Proposed mechanism of P140 on CMA: CMA is a complex multistep process as described elsewhere in detail [17,18,36]. Here we focus on the CMA steps where P140 could play a role. Our results in this paper strongly suggest that P140 inhibits the substrate uptake step of CMA. The co-localization of P140 and HSPA8 was demonstrated earlier [24,27] by fluorescence and electron microscopy. It was not possible, however, to definitively determine that both co-localize into lysosomes or endosomes [27]. P140 interaction with HSPA8 was further demonstrated using surface plasmon resonance experiments and fishing experiments with living cells [23]. We place the effect of P140 in the lysosomal lumen because we have previously shown that incubation of intact lysosomes with P140 in vitro does not modify CMA activity [22]. These findings are in agreement with P140 being internalized by endocytosis and reaching the lysosomal lumen through endolysosome fusion. The co-localization of P140 and LAMP2A has been demonstrated using fluorescence microscopy experiments [24]. Again, due to the resolution of images, it was not possible to definitively determine that that both co-localize within lysosomes. However, the results obtained in this work with a highly purified fraction of lysosomes active for CMA provide strong support of this compartment being the target of P140. Therefore, we hypothesize that the lysosomal P140 encounters and inhibits lysosomal HSP90 and HSPA8, which are responsible of the assembly of LAMP2A multiplex and translocation of CMA substrate, respectively.