FIG. 2.
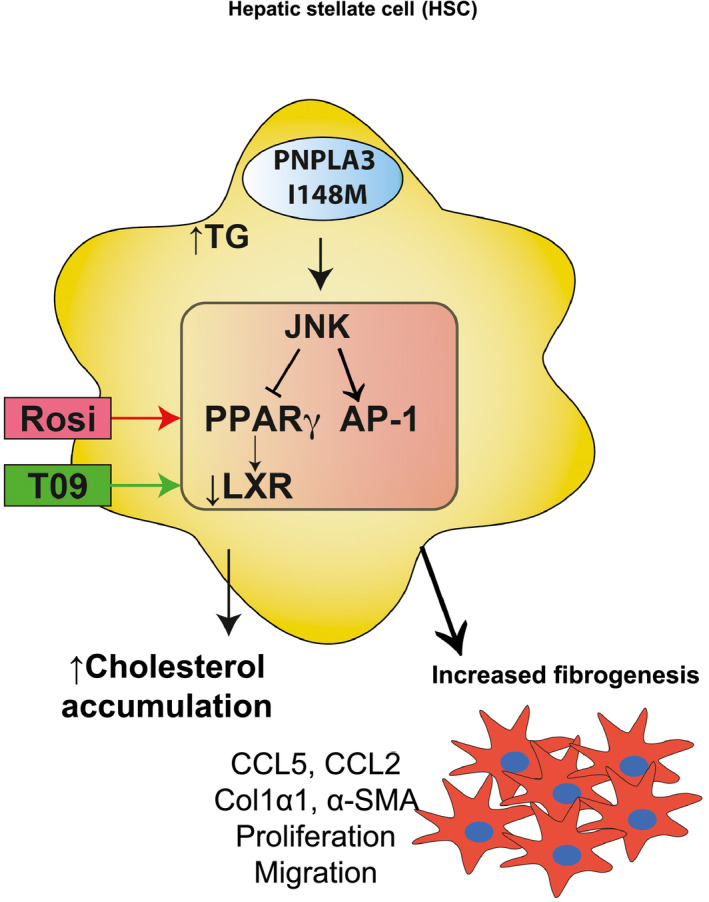
PNPLA3 I148M expressing HSCs show increased fibrogenic features. The PNPLA3 I148M mutation determines increased lipid accumulation, aberrant FFA composition, and reduced retinol content in HSCs. This upregulates JNK signaling, which phosphorylates PPARγ and thereby decreases its transcriptional activity. Lower PPARγ signaling promotes, on one hand, AP‐1–driven production of pro‐inflammatory cytokines, accompanied by immune‐cell recruitment; on the other hand, LXR down‐regulation results in free cholesterol accumulation and worsens profibrogenic features in HSCs. Restoring LXR activity by its pharmacological agonist T0901317 (green arrow), ameliorates cholesterol homeostasis and reduces aberrant activation of HSCs with the PNPLA3 variant, whereas the PPARγ agonist Rosi (red arrow) shows only partial antifibrogenic outcomes. Abbreviations: AP‐1, activator protein 1; CCL, chemokine (C‐C motif) ligand; Col1α1, collagen type 1, α1; FFA, free fatty acid; HSC, hepatic stellate cell; JNK, c‐Jun N‐terminal kinase; LXR, liver X receptor; PNPLA3, patatin‐like phospholipase domain–containing protein 3; PPARγ, peroxisome proliferator–activated receptor γ; Rosi, rosiglitazone; α‐SMA, α‐smooth muscle actin.
